15
Created on January 21, 2023 By  gogorabei
gogorabei chapter3:chemical Equilibrium Quiz1
1 / 30
(1) Equilibrium system is described as dynamic because
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['231'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTE4IjoiMCIsIjkxOSI6IjAiLCI5MjAiOiIwIiwiOTIxIjoiMSJ9fQ==';
2 / 30
(2) The reaction of hydrochloric acid with magnesium is a complete reaction because
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['232'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTIyIjoiMCIsIjkyMyI6IjAiLCI5MjQiOiIxIiwiOTI1IjoiMCJ9fQ==';
3 / 30
(3) --------- is a one of the instantaneous reactions.
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['233'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTI2IjoiMCIsIjkyNyI6IjAiLCI5MjgiOiIxIiwiOTI5IjoiMCJ9fQ==';
4 / 30
(4)---------- is a one of the comparatively slow reactions.
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['234'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTMwIjoiMCIsIjkzMSI6IjAiLCI5MzIiOiIxIiwiOTMzIjoiMCJ9fQ==';
5 / 30
(5) The solution of the reaction of acetic acid with ethyl alcohol turns litmus paper red because
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['235'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTM0IjoiMCIsIjkzNSI6IjAiLCI5MzYiOiIxIiwiOTM3IjoiMCJ9fQ==';
6 / 30
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['236'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTM4IjoiMCIsIjkzOSI6IjEiLCI5NDAiOiIwIiwiOTQxIjoiMCJ9fQ==';
7 / 30
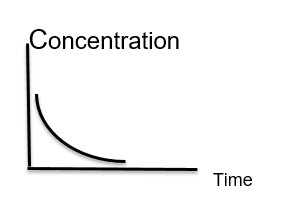
(7) The opposite figure represents -----
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['237'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTQyIjoiMSIsIjk0MyI6IjAiLCI5NDQiOiIwIiwiOTQ1IjoiMCJ9fQ==';
8 / 30
20) The Kc of the reaction:
SnO2+2CO(g) ↔ Sn(s)+2CO2(g)
Is -------------------------
(a) Kc= [CO₂] / [CO]
(b.) Kc= [CO2]2 / [CO]2
(c) Kc= [Sn].[CO₂]² / [SnO2][CO2]2
(d) Kc= [Sn] [CO2]² / [CO]2
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['250'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTk0IjoiMCIsIjk5NSI6IjEiLCI5OTYiOiIwIiwiOTk3IjoiMCJ9fQ==';
9 / 30
(19) The rate of a chemical reaction:
Zn(s) + 2HCl(g) ↔ ZnCl2(aq) +H2(g)
is measured by changing in-------------------
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['249'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTkwIjoiMCIsIjk5MSI6IjAiLCI5OTIiOiIxIiwiOTkzIjoiMCJ9fQ==';
10 / 30
(18) When temperature of the following reaction:
H₂+I2 ↔ 2HI
is raised, the increasing in K1, is less than that of K,2. So, the equilibrium constant Kc ------------
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['248'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTg2IjoiMSIsIjk4NyI6IjAiLCI5ODgiOiIwIiwiOTg5IjoiMCJ9fQ==';
11 / 30
(17) From the Kc value of the reaction:
2SO3(g) ↔ 2SO2(g)+O2 , Kc=1.2x10-4
we can conclude that -----------
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['247'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTgyIjoiMCIsIjk4MyI6IjAiLCI5ODQiOiIwIiwiOTg1IjoiMSJ9fQ==';
12 / 30
(16) From the Kc value for the reaction:
H2(g) + Cl2(g) ↔ 2HCl(g) .Kc=4.4 × 1032
we can conclude that ------
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['246'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTc4IjoiMCIsIjk3OSI6IjEiLCI5ODAiOiIwIiwiOTgxIjoiMCJ9fQ==';
13 / 30
(11) ------- established a law expressing the relationship between the rate of the chemical reaction and concentration of the reactants.
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['241'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTU4IjoiMCIsIjk1OSI6IjAiLCI5NjAiOiIxIiwiOTYxIjoiMCJ9fQ==';
14 / 30
(12) The chemical reaction is in equilibrium when --------
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['242'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTYyIjoiMCIsIjk2MyI6IjEiLCI5NjQiOiIwIiwiOTY1IjoiMCJ9fQ==';
15 / 30
(13) In the following equilibrium reaction:
FeCl3(aq) +3NH SCN(aq) ↔ Fe(SCN)3(aq) + 3NH4Cl(aq)
The rate of reversible reaction is expressed by --------
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['243'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTY2IjoiMCIsIjk2NyI6IjAiLCI5NjgiOiIxIiwiOTY5IjoiMCJ9fQ==';
16 / 30
(14) K1/K2 for an equilibrium reaction is known as K
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['244'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTcwIjoiMSIsIjk3MSI6IjAiLCI5NzIiOiIwIiwiOTczIjoiMCJ9fQ==';
17 / 30
(15) When the value of equilibrium constant is high, it means that ---------
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['245'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTc0IjoiMCIsIjk3NSI6IjEiLCI5NzYiOiIwIiwiOTc3IjoiMCJ9fQ==';
18 / 30
(8) Each of the following affects the chemical equilibrium except the --------- if the volumes of reactants and products are different.
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['238'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTQ2IjoiMCIsIjk0NyI6IjAiLCI5NDgiOiIxIiwiOTQ5IjoiMCJ9fQ==';
19 / 30
(9) The reaction:
N2(g) + 3H2(g) = 2NH3(g) (N=14, H=1]
reaches the equilibrium state when are present in a closed flask. (at S.T.P)
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['239'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTUwIjoiMCIsIjk1MSI6IjAiLCI5NTIiOiIwIiwiOTUzIjoiMSJ9fQ==';
20 / 30
(10) In the reaction: Vegetable oil +H2(g) in presence of Ni /Δ → Margarine (artificial fat)
the nickel used is preferred to be in the form of ......
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['240'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTU0IjoiMCIsIjk1NSI6IjAiLCI5NTYiOiIxIiwiOTU3IjoiMCJ9fQ==';
21 / 30
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['260'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiMTAzNCI6IjAiLCIxMDM1IjoiMCIsIjEwMzYiOiIwIiwiMTAzNyI6IjEifX0=';
22 / 30
(29) On increasing the reactants concentrations of a reaction under equilibrium, a shift in the direction of --------
takes place.
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['259'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiMTAzMCI6IjAiLCIxMDMxIjoiMCIsIjEwMzIiOiIxIiwiMTAzMyI6IjAifX0=';
23 / 30
(21) The concentration of reactants equals the concentration of products at the equilibrium reaction reaction --------
a)Na2O4(g) ↔ 2NO2(g) Kc=0.71
b)H+(aq) +OH-(aq) ↔ H2O(l) Kc= 1x 10 14
c)CO2(g) +H2(g) ↔ CO(g)+ H2O(v) Kc=0.297
d.)SnO2(s) +2H2(g) ↔ Sn(s)+2H2O(v) Kc=1
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['251'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTk4IjoiMCIsIjk5OSI6IjAiLCIxMDAwIjoiMCIsIjEwMDEiOiIxIn19';
24 / 30
(28) On increasing the pressure on a gaseous reaction under equilibrium, a shift in the direction of -------------takes place.
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['258'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiMTAyNiI6IjAiLCIxMDI3IjoiMSIsIjEwMjgiOiIwIiwiMTAyOSI6IjAifX0=';
25 / 30
(27) by increasing the reaction temperature 10°C
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['257'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiMTAyMiI6IjAiLCIxMDIzIjoiMSIsIjEwMjQiOiIwIiwiMTAyNSI6IjAifX0=';
26 / 30
(26) The Kc value of the reaction changes when -----
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['256'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiMTAxOCI6IjAiLCIxMDE5IjoiMSIsIjEwMjAiOiIwIiwiMTAyMSI6IjAifX0=';
27 / 30
(25) The concentration of gases is preferable to expressed by
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['255'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiMTAxNCI6IjAiLCIxMDE1IjoiMCIsIjEwMTYiOiIwIiwiMTAxNyI6IjEifX0=';
28 / 30
(24) The orange colour turns yellow on adding -------- to the follwing reaction:
2CrO42- (aq) + 2H3O+(aq) ↔ Cr2O72- + 3H2O(l)
Yellow orange
(a) KNO3 (b.) NaOH
(c)NH4NO3 (d)CH3COOH
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['254'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiMTAxMCI6IjAiLCIxMDExIjoiMSIsIjEwMTIiOiIwIiwiMTAxMyI6IjAifX0=';
29 / 30
(23) The Kc value equals Kp for the reaction
(a) PCl5(g) ↔ PCl3(g) +Cl2(g)
(b)2SO2(g)+O2(g) ↔ 2SO3(g)
(c.) H2(g) + I2(g) ↔ 2HI(g)
(d) N2(g) +3H2(g) ↔ 2NH3(g)
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['253'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiMTAwNiI6IjAiLCIxMDA3IjoiMCIsIjEwMDgiOiIxIiwiMTAwOSI6IjAifX0=';
30 / 30
(22) Which of the following changes increases the kinetic energy of the reactant molecules ? -------------------
(a) The decreasing of concentration
(b.) The increasing of temperature.
(c) The increasing of surface area.
(d) The increasing of concentration.
if(typeof window.quizOptions_12 === 'undefined'){ window.quizOptions_12 = []; } window.quizOptions_12['252'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiMTAwMiI6IjAiLCIxMDAzIjoiMSIsIjEwMDQiOiIwIiwiMTAwNSI6IjAifX0=';
Your score is
The average score is 34%
div#ays-quiz-container-12 * { box-sizing: border-box; } /* Styles for Internet Explorer start */ #ays-quiz-container-12 #ays_finish_quiz_12 { } /* Styles for Quiz container */ #ays-quiz-container-12{ min-height: 350px; width:400px; background-color:#fff; background-position:center center;border-radius:0px 0px 0px 0px;box-shadow: 0px 0px 15px 1px rgba(0,0,0,0.4);border: none;} /* Styles for questions */ #ays-quiz-container-12 #ays_finish_quiz_12 div.step { min-height: 350px; } /* Styles for text inside quiz container */ #ays-quiz-container-12 .ays-start-page *:not(input), #ays-quiz-container-12 .ays_question_hint, #ays-quiz-container-12 label[for^="ays-answer-"], #ays-quiz-container-12 #ays_finish_quiz_12 p, #ays-quiz-container-12 #ays_finish_quiz_12 .ays-fs-title, #ays-quiz-container-12 .ays-fs-subtitle, #ays-quiz-container-12 .logged_in_message, #ays-quiz-container-12 .ays_score_message, #ays-quiz-container-12 .ays_message{ color: #000; outline: none; } #ays-quiz-container-12 .ays-quiz-password-message-box, #ays-quiz-container-12 .ays-quiz-question-note-message-box, #ays-quiz-container-12 .ays_quiz_question, #ays-quiz-container-12 .ays_quiz_question *:not([class^='enlighter']) { color: #000; } #ays-quiz-container-12 textarea, #ays-quiz-container-12 input::first-letter, #ays-quiz-container-12 select::first-letter, #ays-quiz-container-12 option::first-letter { color: initial !important; } #ays-quiz-container-12 p::first-letter:not(.ays_no_questions_message) { color: #000 !important; background-color: transparent !important; font-size: inherit !important; font-weight: inherit !important; float: none !important; line-height: inherit !important; margin: 0 !important; padding: 0 !important; } #ays-quiz-container-12 .select2-container, #ays-quiz-container-12 .ays-field * { font-size: 15px !important; } #ays-quiz-container-12 .ays_quiz_question p { font-size: 16px; } #ays-quiz-container-12 .ays-fs-subtitle p { text-align: center ; } #ays-quiz-container-12 .ays_quiz_question { text-align: center ; margin-bottom: 10px; } #ays-quiz-container-12 .ays_quiz_question pre { max-width: 100%; white-space: break-spaces; } #ays-quiz-container-12 .ays-quiz-timer p { font-size: 16px; } #ays-quiz-container-12 section.ays_quiz_redirection_timer_container hr, #ays-quiz-container-12 section.ays_quiz_timer_container hr { margin: 0; } #ays-quiz-container-12 section.ays_quiz_timer_container.ays_quiz_timer_red_warning .ays-quiz-timer { color: red; } #ays-quiz-container-12 .ays_thank_you_fs p { text-align: center; } #ays-quiz-container-12 .ays_quiz_results_page .ays_score span { visibility: visible; } #ays-quiz-container-12 input[type='button'], #ays-quiz-container-12 input[type='submit'] { color: #000000 !important; } #ays-quiz-container-12 input[type='button']{ outline: none; } #ays-quiz-container-12 .information_form input[type='text'], #ays-quiz-container-12 .information_form input[type='url'], #ays-quiz-container-12 .information_form input[type='number'], #ays-quiz-container-12 .information_form input[type='email'], #ays-quiz-container-12 .information_form input[type='checkbox'], #ays-quiz-container-12 .information_form input[type='tel'], #ays-quiz-container-12 .information_form textarea, #ays-quiz-container-12 .information_form select, #ays-quiz-container-12 .information_form option { color: initial !important; outline: none; background-image: unset; } #ays-quiz-container-12 .wrong_answer_text{ color:#ff4d4d; } #ays-quiz-container-12 .right_answer_text{ color:#33cc33; } #ays-quiz-container-12 .wrong_answer_text p { font-size:16px; } #ays-quiz-container-12 .ays_questtion_explanation p { font-size:16px; } #ays-quiz-container-12 .right_answer_text p { font-size:16px; } #ays-quiz-container-12 .ays-quiz-question-note-message-box p { font-size:14px; } #ays-quiz-container-12 .ays-quiz-question-note-message-box * { text-transform:none; } #ays-quiz-container-12 .ays_cb_and_a, #ays-quiz-container-12 .ays_cb_and_a * { color: rgb(0,0,0); text-align: center; } /* Quiz textarea height */ #ays-quiz-container-12 textarea { height: 100px; min-height: 100px; } /* Quiz rate and passed users count */ #ays-quiz-container-12 .ays_quizn_ancnoxneri_qanak, #ays-quiz-container-12 .ays_quiz_rete_avg { color:#fff !important; background-color:#000; } #ays-quiz-container-12 .ays-questions-container > .ays_quizn_ancnoxneri_qanak { padding: 5px 20px; } #ays-quiz-container-12 div.for_quiz_rate.ui.star.rating .icon { color: rgba(0,0,0,0.35); } #ays-quiz-container-12 .ays_quiz_rete_avg div.for_quiz_rate_avg.ui.star.rating .icon { color: rgba(255,255,255,0.5); } #ays-quiz-container-12 .ays_quiz_rete .ays-quiz-rate-link-box .ays-quiz-rate-link { color: #000; } /* Loaders */ #ays-quiz-container-12 div.lds-spinner, #ays-quiz-container-12 div.lds-spinner2 { color: #000; } #ays-quiz-container-12 div.lds-spinner div:after, #ays-quiz-container-12 div.lds-spinner2 div:after { background-color: #000; } #ays-quiz-container-12 .lds-circle, #ays-quiz-container-12 .lds-facebook div, #ays-quiz-container-12 .lds-ellipsis div{ background: #000; } #ays-quiz-container-12 .lds-ripple div{ border-color: #000; } #ays-quiz-container-12 .lds-dual-ring::after, #ays-quiz-container-12 .lds-hourglass::after{ border-color: #000 transparent #000 transparent; } /* Stars */ #ays-quiz-container-12 .ui.rating .icon, #ays-quiz-container-12 .ui.rating .icon:before { } /* Progress bars */ #ays-quiz-container-12 #ays_finish_quiz_12 .ays-progress { border-color: rgba(0,0,0,0.8); } #ays-quiz-container-12 #ays_finish_quiz_12 .ays-progress-bg { background-color: rgba(0,0,0,0.3); } #ays-quiz-container-12 .ays-progress-value { color: #000; text-align: center; } #ays-quiz-container-12 .ays-progress-bar { background-color: #27AE60; } #ays-quiz-container-12 .ays-question-counter .ays-live-bar-wrap { direction:ltr !important; } #ays-quiz-container-12 .ays-live-bar-fill{ color: #000; border-bottom: 2px solid rgba(0,0,0,0.8); text-shadow: 0px 0px 5px #fff; } #ays-quiz-container-12 .ays-live-bar-fill.ays-live-fourth, #ays-quiz-container-12 .ays-live-bar-fill.ays-live-third, #ays-quiz-container-12 .ays-live-bar-fill.ays-live-second { text-shadow: unset; } #ays-quiz-container-12 .ays-live-bar-percent{ display:none; } #ays-quiz-container-12 #ays_finish_quiz_12 .ays_average { text-align: center; } /* Music, Sound */ #ays-quiz-container-12 .ays_music_sound { color:rgb(0,0,0); } /* Dropdown questions scroll bar */ #ays-quiz-container-12 blockquote { border-left-color: #000 !important; } /* Quiz Password */ #ays-quiz-container-12 .ays-start-page > input[id^='ays_quiz_password_val_'], #ays-quiz-container-12 .ays-quiz-password-toggle-visibility-box { width: 100%; } /* Question hint */ #ays-quiz-container-12 .ays_question_hint_container .ays_question_hint_text { background-color:#fff; box-shadow: 0 0 15px 3px rgba(0,0,0,0.6); max-width: 270px; } #ays-quiz-container-12 .ays_question_hint_container .ays_question_hint_text p { max-width: unset; } #ays-quiz-container-12 .ays_questions_hint_max_width_class { max-width: 80%; } /* Information form */ #ays-quiz-container-12 .ays-form-title{ color:rgb(0,0,0); } /* Quiz timer */ #ays-quiz-container-12 div.ays-quiz-redirection-timer, #ays-quiz-container-12 div.ays-quiz-timer{ color: #000; text-align: center; } #ays-quiz-container-12 div.ays-quiz-timer.ays-quiz-message-before-timer:before { font-weight: 500; } /* Quiz title / transformation */ #ays-quiz-container-12 .ays-fs-title{ text-transform: uppercase; font-size: 21px; text-align: center; text-shadow: none; } /* Quiz buttons */ #ays-quiz-container-12 .ays_arrow { color:#000000!important; } #ays-quiz-container-12 input#ays-submit, #ays-quiz-container-12 #ays_finish_quiz_12 .action-button, div#ays-quiz-container-12 #ays_finish_quiz_12 .action-button.ays_restart_button, #ays-quiz-container-12 + .ays-quiz-category-selective-main-container .ays-quiz-category-selective-restart-bttn, #ays-quiz-container-12 .ays-quiz-category-selective-submit-bttn { background: none; background-color: #27AE60; color:#000000; font-size: 17px; padding: 10px 20px; border-radius: 3px; height: auto; letter-spacing: 0; box-shadow: unset; } #ays-quiz-container-12 input#ays-submit, #ays-quiz-container-12 #ays_finish_quiz_12 input.action-button, #ays-quiz-container-12 + .ays-quiz-category-selective-main-container .ays-quiz-category-selective-restart-bttn, #ays-quiz-container-12 .ays-quiz-category-selective-submit-bttn { } #ays-quiz-container-12 #ays_finish_quiz_12 .action-button.ays_check_answer { padding: 5px 10px; font-size: 17px !important; } #ays-quiz-container-12 #ays_finish_quiz_12 .action-button.ays_restart_button { white-space: nowrap; padding: 5px 10px; white-space: normal; } #ays-quiz-container-12 input#ays-submit:hover, #ays-quiz-container-12 input#ays-submit:focus, #ays-quiz-container-12 #ays_finish_quiz_12 .action-button:hover, #ays-quiz-container-12 #ays_finish_quiz_12 .action-button:focus, #ays-quiz-container-12 + .ays-quiz-category-selective-main-container .ays-quiz-category-selective-restart-bttn:hover, #ays-quiz-container-12 .ays-quiz-category-selective-submit-bttn:hover { background: none; box-shadow: 0 0 0 2px #000000; background-color: #27AE60; } #ays-quiz-container-12 .ays_restart_button { color: #000000; } #ays-quiz-container-12 .ays_restart_button_p, #ays-quiz-container-12 .ays_buttons_div { justify-content: center; } #ays-quiz-container-12 .ays_finish.action-button{ margin: 10px 5px; } #ays-quiz-container-12 .ays-share-btn.ays-share-btn-branded { color: #fff; display: inline-block; } #ays-quiz-container-12 .ays_quiz_results .ays-field.checked_answer_div.correct_div input:checked+label { background-color: transparent; } /* Question answers */ #ays-quiz-container-12 .ays-field { border-color: #444; border-style: solid; border-width: 1px; box-shadow: none; } /* Answer maximum length of a text field */ #ays-quiz-container-12 .ays_quiz_question_text_message{ color: #000; text-align: left; font-size: 12px; } div#ays-quiz-container-12 div.ays_quiz_question_text_error_message { color: #ff0000; } #ays-quiz-container-12 .ays-quiz-answers .ays-field:hover{ opacity: 1; } #ays-quiz-container-12 #ays_finish_quiz_12 .ays-field { margin-bottom: 10px; } #ays-quiz-container-12 #ays_finish_quiz_12 .ays-field.ays_grid_view_item { width: calc(50% - 5px); } #ays-quiz-container-12 #ays_finish_quiz_12 .ays-field.ays_grid_view_item:nth-child(odd) { margin-right: 5px; } #ays-quiz-container-12 #ays_finish_quiz_12 .ays-field input:checked+label:before { border-color: #27AE60; background: #27AE60; background-clip: content-box; } #ays-quiz-container-12 .ays-quiz-answers div.ays-text-right-answer { color: #000; } /* Questions answer image */ #ays-quiz-container-12 .ays-answer-image { width:50%; } /* Questions answer right/wrong icons */ #ays-quiz-container-12 .ays-field input~label.answered.correct:after{ content: url('https://gogoacademy.net/wp-content/plugins/quiz-maker/public/images/correct.png'); } #ays-quiz-container-12 .ays-field input~label.answered.wrong:after{ content: url('https://gogoacademy.net/wp-content/plugins/quiz-maker/public/images/wrong.png'); } /* Dropdown questions */ #ays-quiz-container-12 #ays_finish_quiz_12 .ays-field .select2-container--default .select2-selection--single { border-bottom: 2px solid #27AE60; background-color: #27AE60; } #ays-quiz-container-12 .ays-field .select2-container--default .select2-selection--single .select2-selection__placeholder, #ays-quiz-container-12 .ays-field .select2-container--default .select2-selection--single .select2-selection__rendered, #ays-quiz-container-12 .ays-field .select2-container--default .select2-selection--single .select2-selection__arrow { color: #ffffff; } #ays-quiz-container-12 .select2-container--default .select2-search--dropdown .select2-search__field:focus, #ays-quiz-container-12 .select2-container--default .select2-search--dropdown .select2-search__field { outline: unset; padding: 0.75rem; } #ays-quiz-container-12 .ays-field .select2-container--default .select2-selection--single .select2-selection__rendered, #ays-quiz-container-12 .select2-container--default .select2-results__option--highlighted[aria-selected] { background-color: #27AE60; } #ays-quiz-container-12 .ays-field .select2-container--default, #ays-quiz-container-12 .ays-field .select2-container--default .selection, #ays-quiz-container-12 .ays-field .select2-container--default .dropdown-wrapper, #ays-quiz-container-12 .ays-field .select2-container--default .select2-selection--single .select2-selection__rendered, #ays-quiz-container-12 .ays-field .select2-container--default .select2-selection--single .select2-selection__rendered .select2-selection__placeholder, #ays-quiz-container-12 .ays-field .select2-container--default .select2-selection--single .select2-selection__arrow, #ays-quiz-container-12 .ays-field .select2-container--default .select2-selection--single .select2-selection__arrow b[role='presentation'] { font-size: 16px !important; } #ays-quiz-container-12 .select2-container--default .select2-results__option { padding: 6px; } /* Dropdown questions scroll bar */ #ays-quiz-container-12 .select2-results__options::-webkit-scrollbar { width: 7px; } #ays-quiz-container-12 .select2-results__options::-webkit-scrollbar-track { background-color: rgba(255,255,255,0.35); } #ays-quiz-container-12 .select2-results__options::-webkit-scrollbar-thumb { transition: .3s ease-in-out; background-color: rgba(0,0,0,0.55); } #ays-quiz-container-12 .select2-results__options::-webkit-scrollbar-thumb:hover { transition: .3s ease-in-out; background-color: rgba(0,0,0,0.85); } /* Audio / Video */ #ays-quiz-container-12 .mejs-container .mejs-time{ box-sizing: unset; } #ays-quiz-container-12 .mejs-container .mejs-time-rail { padding-top: 15px; } #ays-quiz-container-12 .mejs-container .mejs-mediaelement video { margin: 0; } /* Limitation */ #ays-quiz-container-12 .ays-quiz-limitation-count-of-takers { padding: 50px; } #ays-quiz-container-12 div.ays-quiz-results-toggle-block span.ays-show-res-toggle.ays-res-toggle-show, #ays-quiz-container-12 div.ays-quiz-results-toggle-block span.ays-show-res-toggle.ays-res-toggle-hide{ color: #000; } #ays-quiz-container-12 div.ays-quiz-results-toggle-block input:checked + label.ays_switch_toggle { border: 1px solid #000; } #ays-quiz-container-12 div.ays-quiz-results-toggle-block input:checked + label.ays_switch_toggle { border: 1px solid #000; } #ays-quiz-container-12 div.ays-quiz-results-toggle-block input:checked + label.ays_switch_toggle:after{ background: #000; } #ays-quiz-container-12.ays_quiz_elegant_dark div.ays-quiz-results-toggle-block input:checked + label.ays_switch_toggle:after, #ays-quiz-container-12.ays_quiz_rect_dark div.ays-quiz-results-toggle-block input:checked + label.ays_switch_toggle:after{ background: #000; } /* Hestia theme (Version: 3.0.16) | Start */ #ays-quiz-container-12 .mejs-container .mejs-inner .mejs-controls .mejs-button > button:hover, #ays-quiz-container-12 .mejs-container .mejs-inner .mejs-controls .mejs-button > button { box-shadow: unset; background-color: transparent; } #ays-quiz-container-12 .mejs-container .mejs-inner .mejs-controls .mejs-button > button { margin: 10px 6px; } /* Hestia theme (Version: 3.0.16) | End */ /* Go theme (Version: 1.4.3) | Start */ #ays-quiz-container-12 label[for^='ays-answer']:before, #ays-quiz-container-12 label[for^='ays-answer']:before { -webkit-mask-image: unset; mask-image: unset; } #ays-quiz-container-12.ays_quiz_classic_light .ays-field input:checked+label.answered.correct:before, #ays-quiz-container-12.ays_quiz_classic_dark .ays-field input:checked+label.answered.correct:before { background-color: #27AE60 !important; } /* Go theme (Version: 1.4.3) | End */ #ays-quiz-container-12 .ays_quiz_results fieldset.ays_fieldset .ays_quiz_question .wp-video { width: 100% !important; max-width: 100%; } /* Classic Dark / Classic Light */ /* Dropdown questions right/wrong styles */ #ays-quiz-container-12.ays_quiz_classic_dark .correct_div, #ays-quiz-container-12.ays_quiz_classic_light .correct_div{ border-color:green !important; opacity: 1 !important; background-color: rgba(39,174,96,0.4) !important; } #ays-quiz-container-12.ays_quiz_classic_dark .correct_div .selected-field, #ays-quiz-container-12.ays_quiz_classic_light .correct_div .selected-field { padding: 0px 10px 0px 10px; color: green !important; } #ays-quiz-container-12.ays_quiz_classic_dark .wrong_div, #ays-quiz-container-12.ays_quiz_classic_light .wrong_div{ border-color:red !important; opacity: 1 !important; background-color: rgba(243,134,129,0.4) !important; } #ays-quiz-container-12.ays_quiz_classic_dark .ays-field, #ays-quiz-container-12.ays_quiz_classic_light .ays-field { text-align: left; /*margin-bottom: 10px;*/ padding: 0; transition: .3s ease-in-out; } #ays-quiz-container-12 .ays-quiz-close-full-screen { fill: #000; } #ays-quiz-container-12 .ays-quiz-open-full-screen { fill: #000; } #ays-quiz-container-12 .ays_quiz_login_form p{ color: #000; } @media screen and (max-width: 768px){ #ays-quiz-container-12{ max-width: 100%; } #ays-quiz-container-12 .ays_quiz_question p { font-size: 16px; } #ays-quiz-container-12 .select2-container, #ays-quiz-container-12 .ays-field * { font-size: 15px !important; } div#ays-quiz-container-12 input#ays-submit, div#ays-quiz-container-12 #ays_finish_quiz_12 .action-button, div#ays-quiz-container-12 #ays_finish_quiz_12 .action-button.ays_restart_button, #ays-quiz-container-12 + .ays-quiz-category-selective-main-container .ays-quiz-category-selective-restart-bttn, #ays-quiz-container-12 .ays-quiz-category-selective-submit-bttn { font-size: 17px; } /* Quiz title / mobile font size */ div#ays-quiz-container-12 .ays-fs-title { font-size: 21px; } /* Question explanation / mobile font size */ #ays-quiz-container-12 .ays_questtion_explanation p { font-size:16px; } /* Wrong answers / mobile font size */ #ays-quiz-container-12 .wrong_answer_text p { font-size:16px; } /* Right answers / mobile font size */ #ays-quiz-container-12 .right_answer_text p { font-size:16px; } /* Note text / mobile font size */ #ays-quiz-container-12 .ays-quiz-question-note-message-box p { font-size:14px; } } /* Custom css styles */ /* RTL direction styles */ #ays-quiz-container-12 p { margin: 0.625em; } #ays-quiz-container-12 .ays-field.checked_answer_div input:checked+label { background-color: rgba(39,174,96,0.6); } #ays-quiz-container-12.ays_quiz_classic_light .enable_correction .ays-field.checked_answer_div input:checked+label, #ays-quiz-container-12.ays_quiz_classic_dark .enable_correction .ays-field.checked_answer_div input:checked+label { background-color: transparent; } #ays-quiz-container-12 .ays-field.checked_answer_div input:checked+label:hover { background-color: rgba(39,174,96,0.8); } #ays-quiz-container-12 .ays-field:hover label{ background: rgba(39,174,96,0.8); /* border-radius: 4px; */ color: #fff; transition: all .3s; } #ays-quiz-container-12 #ays_finish_quiz_12 .action-button:hover, #ays-quiz-container-12 #ays_finish_quiz_12 .action-button:focus, #ays-quiz-container-12 + .ays-quiz-category-selective-main-container .ays-quiz-category-selective-restart-bttn:hover, #ays-quiz-container-12 .ays-quiz-category-selective-submit-bttn:focus { box-shadow: 0 0 0 2px white, 0 0 0 3px #27AE60; background: #27AE60; } if(typeof aysQuizOptions === 'undefined'){ var aysQuizOptions = []; } aysQuizOptions['12'] = 'eyJxdWl6X3ZlcnNpb24iOiI2LjMuNi44IiwiY29yZV92ZXJzaW9uIjoiNi4wLjMiLCJwaHBfdmVyc2lvbiI6IjcuNC4zMiIsImNvbG9yIjoiIzI3QUU2MCIsImJnX2NvbG9yIjoiI2ZmZiIsInRleHRfY29sb3IiOiIjMDAwIiwiaGVpZ2h0IjozNTAsIndpZHRoIjo0MDAsImVuYWJsZV9sb2dnZWRfdXNlcnMiOiJvZmYiLCJpbmZvcm1hdGlvbl9mb3JtIjoiZGlzYWJsZSIsImZvcm1fbmFtZSI6bnVsbCwiZm9ybV9lbWFpbCI6bnVsbCwiZm9ybV9waG9uZSI6bnVsbCwiaW1hZ2Vfd2lkdGgiOiIiLCJpbWFnZV9oZWlnaHQiOiIiLCJlbmFibGVfY29ycmVjdGlvbiI6Im9mZiIsImVuYWJsZV9wcm9ncmVzc19iYXIiOiJvbiIsImVuYWJsZV9xdWVzdGlvbnNfcmVzdWx0Ijoib24iLCJyYW5kb21pemVfcXVlc3Rpb25zIjoib2ZmIiwicmFuZG9taXplX2Fuc3dlcnMiOiJvZmYiLCJlbmFibGVfcXVlc3Rpb25zX2NvdW50ZXIiOiJvbiIsImVuYWJsZV9yZXN0cmljdGlvbl9wYXNzIjoib2ZmIiwicmVzdHJpY3Rpb25fcGFzc19tZXNzYWdlIjoiIiwidXNlcl9yb2xlIjpbXSwiY3VzdG9tX2NzcyI6IiIsImxpbWl0X3VzZXJzIjoib2ZmIiwibGltaXRhdGlvbl9tZXNzYWdlIjoiIiwicmVkaXJlY3RfdXJsIjoiIiwicmVkaXJlY3Rpb25fZGVsYXkiOjAsImFuc3dlcnNfdmlldyI6Imxpc3QiLCJlbmFibGVfcnRsX2RpcmVjdGlvbiI6Im9mZiIsImVuYWJsZV9sb2dnZWRfdXNlcnNfbWVzc2FnZSI6IiIsInF1ZXN0aW9uc19jb3VudCI6IiIsImVuYWJsZV9xdWVzdGlvbl9iYW5rIjoib2ZmIiwiZW5hYmxlX2xpdmVfcHJvZ3Jlc3NfYmFyIjoib2ZmIiwiZW5hYmxlX3BlcmNlbnRfdmlldyI6Im9mZiIsImVuYWJsZV9hdmVyYWdlX3N0YXRpc3RpY2FsIjoib24iLCJlbmFibGVfbmV4dF9idXR0b24iOiJvbiIsImVuYWJsZV9wcmV2aW91c19idXR0b24iOiJvbiIsImVuYWJsZV9hcnJvd3MiOiJvZmYiLCJ0aW1lcl90ZXh0IjoiIiwicXVpel90aGVtZSI6ImNsYXNzaWNfbGlnaHQiLCJlbmFibGVfc29jaWFsX2J1dHRvbnMiOiJvbiIsInJlc3VsdF90ZXh0IjoiIiwiZW5hYmxlX3Bhc3NfY291bnQiOiJvbiIsImhpZGVfc2NvcmUiOiJvZmYiLCJyYXRlX2Zvcm1fdGl0bGUiOiIiLCJib3hfc2hhZG93X2NvbG9yIjoiIzAwMCIsInF1aXpfYm9yZGVyX3JhZGl1cyI6IjAiLCJxdWl6X2JnX2ltYWdlIjoiIiwicXVpel9ib3JkZXJfd2lkdGgiOiIxIiwicXVpel9ib3JkZXJfc3R5bGUiOiJzb2xpZCIsInF1aXpfYm9yZGVyX2NvbG9yIjoiIzAwMCIsInF1aXpfbG9hZGVyIjoiZGVmYXVsdCIsImNyZWF0ZV9kYXRlIjoiMjAyMy0wMS0yMSAyMjo0Njo0MiIsImF1dGhvciI6IntcImlkXCI6XCIxXCIsXCJuYW1lXCI6XCJnb2dvcmFiZWlcIn0iLCJxdWVzdF9hbmltYXRpb24iOiJzaGFrZSIsImZvcm1fdGl0bGUiOiIiLCJlbmFibGVfYmdfbXVzaWMiOiJvZmYiLCJxdWl6X2JnX211c2ljIjoiIiwiYW5zd2Vyc19mb250X3NpemUiOjE1LCJzaG93X2NyZWF0ZV9kYXRlIjoib24iLCJzaG93X2F1dGhvciI6Im9uIiwiZW5hYmxlX2Vhcmx5X2ZpbmlzaCI6Im9mZiIsImFuc3dlcnNfcndfdGV4dHMiOiJvbl9wYXNzaW5nIiwiZGlzYWJsZV9zdG9yZV9kYXRhIjoib2ZmIiwiZW5hYmxlX2JhY2tncm91bmRfZ3JhZGllbnQiOiJvZmYiLCJiYWNrZ3JvdW5kX2dyYWRpZW50X2NvbG9yXzEiOiIjMDAwIiwiYmFja2dyb3VuZF9ncmFkaWVudF9jb2xvcl8yIjoiI2ZmZiIsInF1aXpfZ3JhZGllbnRfZGlyZWN0aW9uIjoidmVydGljYWwiLCJyZWRpcmVjdF9hZnRlcl9zdWJtaXQiOiJvZmYiLCJzdWJtaXRfcmVkaXJlY3RfdXJsIjoiIiwic3VibWl0X3JlZGlyZWN0X2RlbGF5IjoiMCIsInByb2dyZXNzX2Jhcl9zdHlsZSI6InNlY29uZCIsImVuYWJsZV9leGl0X2J1dHRvbiI6Im9mZiIsImV4aXRfcmVkaXJlY3RfdXJsIjoiIiwiaW1hZ2Vfc2l6aW5nIjoiY292ZXIiLCJxdWl6X2JnX2ltYWdlX3Bvc2l0aW9uIjoiY2VudGVyIGNlbnRlciIsImN1c3RvbV9jbGFzcyI6IiIsImVuYWJsZV9zb2NpYWxfbGlua3MiOiJvZmYiLCJzb2NpYWxfbGlua3MiOnsibGlua2VkaW5fbGluayI6IiIsImZhY2Vib29rX2xpbmsiOiIiLCJ0d2l0dGVyX2xpbmsiOiIiLCJ2a29udGFrdGVfbGluayI6IiIsImluc3RhZ3JhbV9saW5rIjoiIiwieW91dHViZV9saW5rIjoiIiwiYmVoYW5jZV9saW5rIjoiIn0sInNob3dfcXVpel90aXRsZSI6Im9uIiwic2hvd19xdWl6X2Rlc2MiOiJvbiIsInNob3dfbG9naW5fZm9ybSI6Im9mZiIsIm1vYmlsZV9tYXhfd2lkdGgiOiIiLCJsaW1pdF91c2Vyc19ieSI6ImlwIiwiYWN0aXZlX2RhdGVfY2hlY2siOiJvZmYiLCJhY3RpdmVJbnRlcnZhbCI6IjIwMjMtMDEtMjIgMDE6NTc6MTEiLCJkZWFjdGl2ZUludGVydmFsIjoiMjAyMy0wMS0yMiAwMTo1NzoxMSIsImFjdGl2ZV9kYXRlX3ByZV9zdGFydF9tZXNzYWdlIjoiPHA+VGhlIHF1aXogd2lsbCBiZSBhdmFpbGFibGUgc29vbiE8XC9wPiIsImFjdGl2ZV9kYXRlX21lc3NhZ2UiOiI8cD5UaGUgcXVpeiBoYXMgZXhwaXJlZCE8XC9wPiIsImV4cGxhbmF0aW9uX3RpbWUiOiI0IiwiZW5hYmxlX2NsZWFyX2Fuc3dlciI6Im9mZiIsInNob3dfY2F0ZWdvcnkiOiJvZmYiLCJzaG93X3F1ZXN0aW9uX2NhdGVnb3J5Ijoib2ZmIiwiZGlzcGxheV9zY29yZSI6ImJ5X3BlcmNhbnRhZ2UiLCJlbmFibGVfcndfYXNud2Vyc19zb3VuZHMiOiJvZmYiLCJhbnNfcmlnaHRfd3JvbmdfaWNvbiI6ImRlZmF1bHQiLCJxdWl6X2JnX2ltZ19pbl9maW5pc2hfcGFnZSI6Im9mZiIsImZpbmlzaF9hZnRlcl93cm9uZ19hbnN3ZXIiOiJvZmYiLCJhZnRlcl90aW1lcl90ZXh0IjoiIiwiZW5hYmxlX2VudGVyX2tleSI6Im9uIiwiYnV0dG9uc190ZXh0X2NvbG9yIjoiIzAwMDAwMCIsImJ1dHRvbnNfcG9zaXRpb24iOiJjZW50ZXIiLCJzaG93X3F1ZXN0aW9uc19leHBsYW5hdGlvbiI6Im9uX3Jlc3VsdHNfcGFnZSIsImVuYWJsZV9hdWRpb19hdXRvcGxheSI6Im9mZiIsImJ1dHRvbnNfc2l6ZSI6Im1lZGl1bSIsImJ1dHRvbnNfZm9udF9zaXplIjoiMTciLCJidXR0b25zX3dpZHRoIjoiIiwiYnV0dG9uc19sZWZ0X3JpZ2h0X3BhZGRpbmciOiIyMCIsImJ1dHRvbnNfdG9wX2JvdHRvbV9wYWRkaW5nIjoiMTAiLCJidXR0b25zX2JvcmRlcl9yYWRpdXMiOiIzIiwiZW5hYmxlX2xlYXZlX3BhZ2UiOiJvbiIsImVuYWJsZV90YWNrZXJzX2NvdW50Ijoib2ZmIiwidGFja2Vyc19jb3VudCI6IiIsInBhc3Nfc2NvcmUiOjAsInBhc3Nfc2NvcmVfbWVzc2FnZSI6IjxoNCBzdHlsZT1cInRleHQtYWxpZ246IGNlbnRlclwiPkNvbmdyYXR1bGF0aW9ucyE8XC9oND5cclxuPHAgc3R5bGU9XCJ0ZXh0LWFsaWduOiBjZW50ZXJcIj5Zb3UgcGFzc2VkIHRoZSBxdWl6ITxcL3A+IiwiZmFpbF9zY29yZV9tZXNzYWdlIjoiPGg0IHN0eWxlPVwidGV4dC1hbGlnbjogY2VudGVyXCI+T29wcyE8XC9oND5cclxuPHAgc3R5bGU9XCJ0ZXh0LWFsaWduOiBjZW50ZXJcIj5Zb3UgaGF2ZSBub3QgcGFzc2VkIHRoZSBxdWl6ISA8YnIgXC8+XHJcblRyeSBhZ2FpbiE8XC9wPiIsInF1ZXN0aW9uX2ZvbnRfc2l6ZSI6MTYsInF1aXpfd2lkdGhfYnlfcGVyY2VudGFnZV9weCI6InBpeGVscyIsInF1ZXN0aW9uc19oaW50X2ljb25fb3JfdGV4dCI6ImRlZmF1bHQiLCJxdWVzdGlvbnNfaGludF92YWx1ZSI6IiIsImVuYWJsZV9lYXJseV9maW5zaF9jb21maXJtX2JveCI6Im9uIiwiZW5hYmxlX3F1ZXN0aW9uc19vcmRlcmluZ19ieV9jYXQiOiJvZmYiLCJzaG93X3NjaGVkdWxlX3RpbWVyIjoib2ZmIiwic2hvd190aW1lcl90eXBlIjoiY291bnRkb3duIiwicXVpel9sb2FkZXJfdGV4dF92YWx1ZSI6IiIsImhpZGVfY29ycmVjdF9hbnN3ZXJzIjoib2ZmIiwic2hvd19pbmZvcm1hdGlvbl9mb3JtIjoib24iLCJxdWl6X2xvYWRlcl9jdXN0b21fZ2lmIjoiIiwiZGlzYWJsZV9ob3Zlcl9lZmZlY3QiOiJvZmYiLCJxdWl6X2xvYWRlcl9jdXN0b21fZ2lmX3dpZHRoIjoxMDAsInByb2dyZXNzX2xpdmVfYmFyX3N0eWxlIjoiZGVmYXVsdCIsInF1aXpfdGl0bGVfdHJhbnNmb3JtYXRpb24iOiJ1cHBlcmNhc2UiLCJzaG93X2Fuc3dlcnNfbnVtYmVyaW5nIjoibm9uZSIsInF1aXpfaW1hZ2Vfd2lkdGhfYnlfcGVyY2VudGFnZV9weCI6InBpeGVscyIsInF1aXpfaW1hZ2VfaGVpZ2h0IjoiIiwicXVpel9iZ19pbWdfb25fc3RhcnRfcGFnZSI6Im9mZiIsInF1aXpfYm94X3NoYWRvd194X29mZnNldCI6MCwicXVpel9ib3hfc2hhZG93X3lfb2Zmc2V0IjowLCJxdWl6X2JveF9zaGFkb3dfel9vZmZzZXQiOjE1LCJxdWl6X3F1ZXN0aW9uX3RleHRfYWxpZ25tZW50IjoiY2VudGVyIiwicXVpel9hcnJvd190eXBlIjoiZGVmYXVsdCIsInF1aXpfc2hvd193cm9uZ19hbnN3ZXJzX2ZpcnN0Ijoib2ZmIiwicXVpel9kaXNwbGF5X2FsbF9xdWVzdGlvbnMiOiJvZmYiLCJxdWl6X3RpbWVyX3JlZF93YXJuaW5nIjoib2ZmIiwicXVpel9zY2hlZHVsZV90aW1lem9uZSI6IlVUQyswIiwicXVlc3Rpb25zX2hpbnRfYnV0dG9uX3ZhbHVlIjoiIiwicXVpel90YWNrZXJzX21lc3NhZ2UiOiI8cD5UaGlzIHF1aXogaXMgZXhwaXJlZCE8XC9wPiIsInF1aXpfZW5hYmxlX2xpbmtlZGluX3NoYXJlX2J1dHRvbiI6Im9uIiwicXVpel9lbmFibGVfZmFjZWJvb2tfc2hhcmVfYnV0dG9uIjoib24iLCJxdWl6X2VuYWJsZV90d2l0dGVyX3NoYXJlX2J1dHRvbiI6Im9uIiwicXVpel9tYWtlX3Jlc3BvbnNlc19hbm9ueW1vdXMiOiJvZmYiLCJxdWl6X21ha2VfYWxsX3Jldmlld19saW5rIjoib2ZmIiwic2hvd19xdWVzdGlvbnNfbnVtYmVyaW5nIjoibm9uZSIsInF1aXpfbWVzc2FnZV9iZWZvcmVfdGltZXIiOiIiLCJlbmFibGVfcGFzc3dvcmQiOiJvZmYiLCJwYXNzd29yZF9xdWl6IjoiIiwicXVpel9wYXNzd29yZF9tZXNzYWdlIjoiIiwiZW5hYmxlX3NlZV9yZXN1bHRfY29uZmlybV9ib3giOiJvZmYiLCJkaXNwbGF5X2ZpZWxkc19sYWJlbHMiOiJvZmYiLCJlbmFibGVfZnVsbF9zY3JlZW5fbW9kZSI6Im9mZiIsInF1aXpfZW5hYmxlX3Bhc3N3b3JkX3Zpc2liaWxpdHkiOiJvZmYiLCJxdWVzdGlvbl9tb2JpbGVfZm9udF9zaXplIjoxNiwiYW5zd2Vyc19tb2JpbGVfZm9udF9zaXplIjoxNSwic29jaWFsX2J1dHRvbnNfaGVhZGluZyI6IiIsInF1aXpfZW5hYmxlX3Zrb250YWt0ZV9zaGFyZV9idXR0b24iOiJvbiIsImFuc3dlcnNfYm9yZGVyIjoib24iLCJhbnN3ZXJzX2JvcmRlcl93aWR0aCI6MSwiYW5zd2Vyc19ib3JkZXJfc3R5bGUiOiJzb2xpZCIsImFuc3dlcnNfYm9yZGVyX2NvbG9yIjoiIzQ0NCIsInNvY2lhbF9saW5rc19oZWFkaW5nIjoiIiwicXVpel9lbmFibGVfcXVlc3Rpb25fY2F0ZWdvcnlfZGVzY3JpcHRpb24iOiJvZmYiLCJhbnN3ZXJzX21hcmdpbiI6MTAsInF1aXpfbWVzc2FnZV9iZWZvcmVfcmVkaXJlY3RfdGltZXIiOiIiLCJidXR0b25zX21vYmlsZV9mb250X3NpemUiOjE3LCJhbnN3ZXJzX2JveF9zaGFkb3ciOiJvZmYiLCJhbnN3ZXJzX2JveF9zaGFkb3dfY29sb3IiOiIjMDAwIiwicXVpel9hbnN3ZXJfYm94X3NoYWRvd194X29mZnNldCI6MCwicXVpel9hbnN3ZXJfYm94X3NoYWRvd195X29mZnNldCI6MCwicXVpel9hbnN3ZXJfYm94X3NoYWRvd196X29mZnNldCI6MTAsInF1aXpfY3JlYXRlX2F1dGhvciI6MSwicXVpel9lbmFibGVfdGl0bGVfdGV4dF9zaGFkb3ciOiJvZmYiLCJxdWl6X3RpdGxlX3RleHRfc2hhZG93X2NvbG9yIjoiIzMzMyIsInF1aXpfdGl0bGVfdGV4dF9zaGFkb3dfeF9vZmZzZXQiOjIsInF1aXpfdGl0bGVfdGV4dF9zaGFkb3dfeV9vZmZzZXQiOjIsInF1aXpfdGl0bGVfdGV4dF9zaGFkb3dfel9vZmZzZXQiOjIsInF1aXpfc2hvd19vbmx5X3dyb25nX2Fuc3dlcnMiOiJvZmYiLCJxdWl6X3RpdGxlX2ZvbnRfc2l6ZSI6MjEsInF1aXpfdGl0bGVfbW9iaWxlX2ZvbnRfc2l6ZSI6MjEsInF1aXpfcGFzc3dvcmRfd2lkdGgiOiIiLCJxdWl6X3Jldmlld19wbGFjZWhvbGRlcl90ZXh0IjoiIiwicXVpel9tYWtlX3Jldmlld19yZXF1aXJlZCI6Im9mZiIsInF1aXpfZW5hYmxlX3Jlc3VsdHNfdG9nZ2xlIjoib2ZmIiwicmVxdWlyZWRfZmllbGRzIjpudWxsLCJlbmFibGVfdGltZXIiOiJvZmYiLCJlbmFibGVfcXVpel9yYXRlIjoib2ZmIiwiZW5hYmxlX3JhdGVfYXZnIjoib2ZmIiwiZW5hYmxlX2JveF9zaGFkb3ciOiJvbiIsImVuYWJsZV9ib3JkZXIiOiJvZmYiLCJxdWl6X3RpbWVyX2luX3RpdGxlIjoib2ZmIiwiZW5hYmxlX3JhdGVfY29tbWVudHMiOiJvZmYiLCJlbmFibGVfcmVzdGFydF9idXR0b24iOiJvbiIsImF1dG9maWxsX3VzZXJfZGF0YSI6Im9mZiIsInRpbWVyIjoxMDAsInF1aXpfd2FpdGluZ190aW1lIjoib2ZmIiwicXVpel9lbmFibGVfcXVpel9jYXRlZ29yeV9kZXNjcmlwdGlvbiI6Im9mZiIsInF1aXpfZW5hYmxlX3VzZXJfY1x1MDU3MG9vc2luZ19hbm9ueW1vdXNfYXNzZXNzbWVudCI6Im9mZiIsInN1Ym1pdF9yZWRpcmVjdF9hZnRlciI6IiIsInJ3X2Fuc3dlcnNfc291bmRzIjpmYWxzZSwiaWQiOiIxMiIsInRpdGxlIjoiY2hhcHRlcjM6Y2hlbWljYWwgRXF1aWxpYnJpdW0gUXVpejEiLCJkZXNjcmlwdGlvbiI6IiIsInF1aXpfaW1hZ2UiOiIiLCJxdWl6X2NhdGVnb3J5X2lkIjoiNCIsInF1ZXN0aW9uX2lkcyI6IjIzMSwyMzIsMjMzLDIzNCwyMzUsMjM2LDIzNywyNTAsMjQ5LDI0OCwyNDcsMjQ2LDI0MSwyNDIsMjQzLDI0NCwyNDUsMjM4LDIzOSwyNDAsMjYwLDI1OSwyNTEsMjU4LDI1NywyNTYsMjU1LDI1NCwyNTMsMjUyIiwib3JkZXJpbmciOiIxMiIsInB1Ymxpc2hlZCI6IjEiLCJpbnRlcnZhbHMiOm51bGwsInF1aXpfdXJsIjpudWxsLCJxdWl6X2FuaW1hdGlvbl90b3AiOjEwMCwicXVpel9lbmFibGVfYW5pbWF0aW9uX3RvcCI6Im9uIn0=';