Created on January 15, 2023 By  gogorabei
gogorabeichapter5: Organic chemistry
organic compounds and classification of Hydrocarbons
1 / 50
Category: chapter5:organic chemistery
(43) The molecular formula C6H12 is a formula of -------------
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['225'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODk2IjoiMCIsIjg5NSI6IjAiLCI4OTciOiIxIiwiODk0IjoiMCJ9fQ==';
2 / 50
Category: chapter5:organic chemistery
(42) Propyne (C2H4) is an example of ---------------
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['224'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODkzIjoiMCIsIjg5MiI6IjEiLCI4OTEiOiIwIiwiODkwIjoiMCJ9fQ==';
3 / 50
Category: chapter5:organic chemistery
(47) The molecular formula of naphthalene is....
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['230'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTE3IjoiMCIsIjkxNiI6IjEiLCI5MTQiOiIwIiwiOTE1IjoiMCJ9fQ==';
4 / 50
Category: chapter5:organic chemistery
(41) The organic compounds with formula C2H4, is ------------ compound
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['223'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODg2IjoiMCIsIjg4NyI6IjAiLCI4ODkiOiIxIiwiODg4IjoiMCJ9fQ==';
5 / 50
Category: chapter5:organic chemistery
(10) The bonds in organic compounds are .......bonds.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['212'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODQzIjoiMCIsIjg0NSI6IjAiLCI4NDIiOiIxIiwiODQ0IjoiMCJ9fQ==';
6 / 50
Category: chapter5:organic chemistery
(36) The phenomenon that many organic compounds have the same molecular formula is called............
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['217'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODY1IjoiMCIsIjg2MyI6IjAiLCI4NjQiOiIxIiwiODYyIjoiMCJ9fQ==';
7 / 50
Category: chapter5:organic chemistery
(35) The formula that shows the number and the type of atoms only in the molecule formula.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['216'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODYxIjoiMCIsIjg1OSI6IjEiLCI4NjAiOiIwIiwiODU4IjoiMCJ9fQ==';
8 / 50
Category: chapter5:organic chemistery
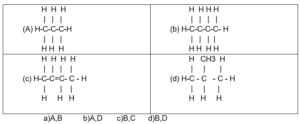
(40) Any combination of the following hydrocarbons represents the isomer to the another one ?
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['221'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODgxIjoiMSIsIjg3OSI6IjAiLCI4ODAiOiIwIiwiODc4IjoiMCJ9fQ==';
9 / 50
Category: chapter5:organic chemistery
(37) All organic compounds contain------ in their structure.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['218'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODY2IjoiMCIsIjg2OCI6IjAiLCI4NjciOiIwIiwiODY5IjoiMSJ9fQ==';
10 / 50
Category: chapter5:organic chemistery
(44) On burning an organic substance with copper oxide, ----------- are produced.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['227'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTA0IjoiMCIsIjkwMyI6IjAiLCI5MDUiOiIwIiwiOTAyIjoiMSJ9fQ==';
11 / 50
Category: chapter5:organic chemistery
30)The carbon atoms can combine with each other by............bonds.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['210'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODM2IjoiMCIsIjgzNCI6IjAiLCI4MzciOiIxIiwiODM1IjoiMCJ9fQ==';
12 / 50
Category: chapter5:organic chemistery
(40) The hydrocarbons with the molecular formula CnH2n+2 are
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['222'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODg1IjoiMCIsIjg4MiI6IjAiLCI4ODMiOiIwIiwiODg0IjoiMSJ9fQ==';
13 / 50
Category: chapter5:organic chemistery
(32) The boiling and melting points of organic compounds are
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['213'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODQ4IjoiMSIsIjg0NyI6IjAiLCI4NDkiOiIwIiwiODQ2IjoiMCJ9fQ==';
14 / 50
Category: chapter5:organic chemistery
(2) The compounds which are extracted from mineral sources in the Earth.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['182'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzI1IjoiMCIsIjcyNCI6IjAiLCI3MjMiOiIxIiwiNzIyIjoiMCJ9fQ==';
15 / 50
Category: chapter5:organic chemistery
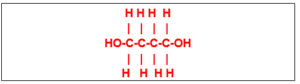
(38) The compound with the structural formula: Follows a group of the compounds
whose general formula is
(a) C2nH5nOn (c) CnH2n+2O2
(b)CnH2nO2 (d) CnH2n-2O2
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['219'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODcwIjoiMCIsIjg3MiI6IjEiLCI4NzMiOiIwIiwiODcxIjoiMCJ9fQ==';
16 / 50
Category: chapter5:organic chemistery
(33) Reactions between organic compounds are slow because they take place between..........
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['214'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODUxIjoiMSIsIjg1MiI6IjAiLCI4NTAiOiIwIiwiODUzIjoiMCJ9fQ==';
17 / 50
Category: chapter5:organic chemistery
(39) The corresponding two compounds are similar in-------- and differ in ------------
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['220'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODc1IjoiMCIsIjg3NyI6IjAiLCI4NzQiOiIwIiwiODc2IjoiMSJ9fQ==';
18 / 50
Category: chapter5:organic chemistery
(31) Most of organic compounds soluble in ------
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['211'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODM4IjoiMCIsIjg0MSI6IjAiLCI4MzkiOiIwIiwiODQwIjoiMSJ9fQ==';
19 / 50
Category: chapter5:organic chemistery
(45) Benzene and naphthalene are examples of hydrocarbons.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['228'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTA2IjoiMCIsIjkwOCI6IjAiLCI5MDciOiIwIiwiOTA5IjoiMSJ9fQ==';
20 / 50
Category: chapter5:organic chemistery
(44) We can detect the presence of water by using ---------------
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['226'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODk5IjoiMCIsIjg5OCI6IjAiLCI5MDEiOiIwIiwiOTAwIjoiMSJ9fQ==';
21 / 50
Category: chapter5:organic chemistery
(46) The molecular formula of aromatic benzene is ----------
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['229'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiOTEyIjoiMCIsIjkxMSI6IjAiLCI5MTAiOiIxIiwiOTEzIjoiMCJ9fQ==';
22 / 50
Category: chapter1 Transition Elements
(6) The basic element in all the organic compounds.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['186'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzQxIjoiMSIsIjczOSI6IjAiLCI3MzgiOiIwIiwiNzQwIjoiMCJ9fQ==';
23 / 50
Category: chapter1 Transition Elements
(26) The organic compound which is obtained on heating ammonium cyanate is ------
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['205'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODE1IjoiMSIsIjgxNiI6IjAiLCI4MTciOiIwIiwiODE0IjoiMCJ9fQ==';
24 / 50
Category: chapter1 Transition Elements
(1) The compounds which have been thought that they are extracted from plants and animals only.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['181'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzIwIjoiMCIsIjcxOCI6IjEiLCI3MTkiOiIwIiwiNzIxIjoiMCJ9fQ==';
25 / 50
Category: chapter1 Transition Elements
(3) A theory which considered that organic compounds are formed in the cells of living organisms only.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['183'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzI3IjoiMSIsIjcyNiI6IjAiLCI3MjkiOiIwIiwiNzI4IjoiMCJ9fQ==';
26 / 50
Category: chapter1 Transition Elements
(21) The organic compounds in which all the corners of the ring have carbon atoms only
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['200'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzk0IjoiMSIsIjc5NiI6IjAiLCI3OTUiOiIwIiwiNzk3IjoiMCJ9fQ==';
27 / 50
Category: chapter1 Transition Elements
(28) The organic chemistry focused on the study of carbon element with exception of
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['207'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODI1IjoiMSIsIjgyMiI6IjAiLCI4MjMiOiIwIiwiODI0IjoiMCJ9fQ==';
28 / 50
Category: chapter1 Transition Elements
(5) The organic compound that was obtained on heating aqueous solutions of ammonium chloride and silver cyanate.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['185'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzM2IjoiMCIsIjczNSI6IjAiLCI3MzQiOiIwIiwiNzM3IjoiMSJ9fQ==';
29 / 50
Category: chapter1 Transition Elements
(7) The branch of Chemistry that focused on the study of carbon element with exception of carbon oxides, carbonate and cyanide salts.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['187'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzQ0IjoiMSIsIjc0MiI6IjAiLCI3NDUiOiIwIiwiNzQzIjoiMCJ9fQ==';
30 / 50
Category: chapter1 Transition Elements
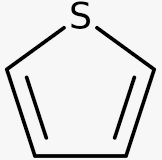
26) The carbon atoms of thiophene are bonded with sulphur in the form of compound.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['208'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODI3IjoiMCIsIjgyOSI6IjAiLCI4MjYiOiIwIiwiODI4IjoiMSJ9fQ==';
31 / 50
Category: chapter1 Transition Elements
(11) The substance which added to the organic compound to detect the presence of carbon and hydrogen.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['191'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzU5IjoiMCIsIjc2MSI6IjEiLCI3NTgiOiIwIiwiNzYwIjoiMCJ9fQ==';
32 / 50
Category: chapter1 Transition Elements
(18) Cyclic hydrocarbons, their general molecular formula are CnH2n+2
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['197'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzgzIjoiMCIsIjc4MiI6IjAiLCI3ODQiOiIxIiwiNzg1IjoiMCJ9fQ==';
33 / 50
Category: chapter1 Transition Elements
(27) All the following compounds are organic compounds except
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['206'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODIwIjoiMCIsIjgxOSI6IjAiLCI4MjEiOiIxIiwiODE4IjoiMCJ9fQ==';
34 / 50
Category: chapter1 Transition Elements
(20) Unsaturated aliphatic hydrocarbons, their general molecular formula are: CnH2n-2
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['199'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzkwIjoiMCIsIjc5MSI6IjAiLCI3OTIiOiIxIiwiNzkzIjoiMCJ9fQ==';
35 / 50
Category: chapter1 Transition Elements
25) The scientist who destroyed the vital force theory is.....
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['204'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODEwIjoiMSIsIjgxMiI6IjAiLCI4MTEiOiIwIiwiODEzIjoiMCJ9fQ==';
36 / 50
Category: chapter1 Transition Elements
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['209'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODMzIjoiMCIsIjgzMSI6IjEiLCI4MzIiOiIwIiwiODMwIjoiMCJ9fQ==';
37 / 50
Category: chapter1 Transition Elements
(13) The saturated open chain aliphatic hydrocarbons.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['193'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzY2IjoiMCIsIjc2OCI6IjEiLCI3NjciOiIwIiwiNzY5IjoiMCJ9fQ==';
38 / 50
Category: chapter1 Transition Elements
(16) The unsaturated open chain aliphatic hydrocarbons, which are characterized by the presence of a triple bond at least in the carbon chain.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['196'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzgxIjoiMCIsIjc3OSI6IjAiLCI3ODAiOiIxIiwiNzc4IjoiMCJ9fQ==';
39 / 50
Category: chapter1 Transition Elements
(9) The formula which indicates the type of linkage between the atoms by the covalent bonds.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['189'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzUxIjoiMSIsIjc1MiI6IjAiLCI3NTMiOiIwIiwiNzUwIjoiMCJ9fQ==';
40 / 50
Category: chapter1 Transition Elements
(12) The organic compounds which consist of carbon and hydrogen only.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['192'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzY1IjoiMCIsIjc2NCI6IjAiLCI3NjMiOiIwIiwiNzYyIjoiMSJ9fQ==';
41 / 50
Category: chapter1 Transition Elements
(15) The unsaturated open chain aliphatic hydrocarbons, which are characterized by the presence of a double bond at least in the carbon chain.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['195'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzc0IjoiMSIsIjc3NSI6IjAiLCI3NzciOiIwIiwiNzc2IjoiMCJ9fQ==';
42 / 50
Category: chapter1 Transition Elements
24)The scientist who classified the compounds into organic and inorganic compounds
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['203'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODA3IjoiMSIsIjgwNiI6IjAiLCI4MDkiOiIwIiwiODA4IjoiMCJ9fQ==';
43 / 50
Category: chapter1 Transition Elements
(22) The organic compounds in which the corners of the ring have carbon atoms and other atoms of different elements.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['201'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzk5IjoiMSIsIjc5OCI6IjAiLCI4MDEiOiIwIiwiODAwIjoiMCJ9fQ==';
44 / 50
Category: chapter1 Transition Elements
(4) The first organic compound that was prepared in Lab. not in the living cells
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['184'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzMyIjoiMCIsIjczMyI6IjAiLCI3MzAiOiIwIiwiNzMxIjoiMSJ9fQ==';
45 / 50
Category: chapter1 Transition Elements
23)Classify the following compounds into : (alkanes – alkenes - alkynes):
(1) C3H8 (2) C7H₁₂ (3) C6H12 (4) C4H10 (5) C40H82 (6) C5H12
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['202'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiODAzIjoiMCIsIjgwNSI6IjAiLCI4MDQiOiIwIiwiODAyIjoiMSJ9fQ==';
46 / 50
Category: chapter1 Transition Elements
(19) Unsaturated aliphatic hydrocarbons, their general molecular formula are : CnH2n
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['198'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzg5IjoiMSIsIjc4OCI6IjAiLCI3ODciOiIwIiwiNzg2IjoiMCJ9fQ==';
47 / 50
Category: chapter1 Transition Elements
(8) The formula which indicates the kind and number of the atoms of each element in
the compound.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['188'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzQ5IjoiMCIsIjc0NiI6IjEiLCI3NDgiOiIwIiwiNzQ3IjoiMCJ9fQ==';
48 / 50
Category: chapter1 Transition Elements
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['180'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzE3IjoiMCIsIjcxNCI6IjAiLCI3MTUiOiIwIiwiNzE2IjoiMSJ9fQ==';
49 / 50
Category: chapter1 Transition Elements
(14) The saturated cyclic aliphatic hydrocarbons.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['194'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzczIjoiMCIsIjc3MiI6IjAiLCI3NzAiOiIwIiwiNzcxIjoiMSJ9fQ==';
50 / 50
Category: chapter1 Transition Elements
(10) It is a phenomenon that many organic compounds have the same molecular formula but differ in structural formula.
if(typeof window.quizOptions_11 === 'undefined'){ window.quizOptions_11 = []; } window.quizOptions_11['190'] = 'eyJxdWVzdGlvbl9hbnN3ZXIiOnsiNzU2IjoiMCIsIjc1NCI6IjEiLCI3NTUiOiIwIiwiNzU3IjoiMCJ9fQ==';
Your score is
The average score is 32%
div#ays-quiz-container-11 * { box-sizing: border-box; } /* Styles for Internet Explorer start */ #ays-quiz-container-11 #ays_finish_quiz_11 { } /* Styles for Quiz container */ #ays-quiz-container-11{ min-height: 350px; width:400px; background-color:#fff; background-position:center center;border-radius:0px;box-shadow: 0px 0px 15px 1px rgba(0,0,0,0.4);border: none;} /* Styles for questions */ #ays-quiz-container-11 #ays_finish_quiz_11 div.step { min-height: 350px; } /* Styles for text inside quiz container */ #ays-quiz-container-11 .ays-start-page *:not(input), #ays-quiz-container-11 .ays_question_hint, #ays-quiz-container-11 label[for^="ays-answer-"], #ays-quiz-container-11 #ays_finish_quiz_11 p, #ays-quiz-container-11 #ays_finish_quiz_11 .ays-fs-title, #ays-quiz-container-11 .ays-fs-subtitle, #ays-quiz-container-11 .logged_in_message, #ays-quiz-container-11 .ays_score_message, #ays-quiz-container-11 .ays_message{ color: #000; outline: none; } #ays-quiz-container-11 .ays-quiz-password-message-box, #ays-quiz-container-11 .ays-quiz-question-note-message-box, #ays-quiz-container-11 .ays_quiz_question, #ays-quiz-container-11 .ays_quiz_question *:not([class^='enlighter']) { color: #000; } #ays-quiz-container-11 textarea, #ays-quiz-container-11 input::first-letter, #ays-quiz-container-11 select::first-letter, #ays-quiz-container-11 option::first-letter { color: initial !important; } #ays-quiz-container-11 p::first-letter:not(.ays_no_questions_message) { color: #000 !important; background-color: transparent !important; font-size: inherit !important; font-weight: inherit !important; float: none !important; line-height: inherit !important; margin: 0 !important; padding: 0 !important; } #ays-quiz-container-11 .select2-container, #ays-quiz-container-11 .ays-field * { font-size: 15px !important; } #ays-quiz-container-11 .ays_quiz_question p { font-size: 16px; } #ays-quiz-container-11 .ays-fs-subtitle p { text-align: center ; } #ays-quiz-container-11 .ays_quiz_question { text-align: center ; margin-bottom: 10px; } #ays-quiz-container-11 .ays_quiz_question pre { max-width: 100%; white-space: break-spaces; } #ays-quiz-container-11 .ays-quiz-timer p { font-size: 16px; } #ays-quiz-container-11 section.ays_quiz_redirection_timer_container hr, #ays-quiz-container-11 section.ays_quiz_timer_container hr { margin: 0; } #ays-quiz-container-11 section.ays_quiz_timer_container.ays_quiz_timer_red_warning .ays-quiz-timer { color: red; } #ays-quiz-container-11 .ays_thank_you_fs p { text-align: center; } #ays-quiz-container-11 .ays_quiz_results_page .ays_score span { visibility: visible; } #ays-quiz-container-11 input[type='button'], #ays-quiz-container-11 input[type='submit'] { color: #000000 !important; } #ays-quiz-container-11 input[type='button']{ outline: none; } #ays-quiz-container-11 .information_form input[type='text'], #ays-quiz-container-11 .information_form input[type='url'], #ays-quiz-container-11 .information_form input[type='number'], #ays-quiz-container-11 .information_form input[type='email'], #ays-quiz-container-11 .information_form input[type='checkbox'], #ays-quiz-container-11 .information_form input[type='tel'], #ays-quiz-container-11 .information_form textarea, #ays-quiz-container-11 .information_form select, #ays-quiz-container-11 .information_form option { color: initial !important; outline: none; background-image: unset; } #ays-quiz-container-11 .wrong_answer_text{ color:#ff4d4d; } #ays-quiz-container-11 .right_answer_text{ color:#33cc33; } #ays-quiz-container-11 .wrong_answer_text p { font-size:16px; } #ays-quiz-container-11 .ays_questtion_explanation p { font-size:16px; } #ays-quiz-container-11 .right_answer_text p { font-size:16px; } #ays-quiz-container-11 .ays-quiz-question-note-message-box p { font-size:14px; } #ays-quiz-container-11 .ays-quiz-question-note-message-box * { text-transform:none; } #ays-quiz-container-11 .ays_cb_and_a, #ays-quiz-container-11 .ays_cb_and_a * { color: rgb(0,0,0); text-align: center; } /* Quiz textarea height */ #ays-quiz-container-11 textarea { height: 100px; min-height: 100px; } /* Quiz rate and passed users count */ #ays-quiz-container-11 .ays_quizn_ancnoxneri_qanak, #ays-quiz-container-11 .ays_quiz_rete_avg { color:#fff !important; background-color:#000; } #ays-quiz-container-11 .ays-questions-container > .ays_quizn_ancnoxneri_qanak { padding: 5px 20px; } #ays-quiz-container-11 div.for_quiz_rate.ui.star.rating .icon { color: rgba(0,0,0,0.35); } #ays-quiz-container-11 .ays_quiz_rete_avg div.for_quiz_rate_avg.ui.star.rating .icon { color: rgba(255,255,255,0.5); } #ays-quiz-container-11 .ays_quiz_rete .ays-quiz-rate-link-box .ays-quiz-rate-link { color: #000; } /* Loaders */ #ays-quiz-container-11 div.lds-spinner, #ays-quiz-container-11 div.lds-spinner2 { color: #000; } #ays-quiz-container-11 div.lds-spinner div:after, #ays-quiz-container-11 div.lds-spinner2 div:after { background-color: #000; } #ays-quiz-container-11 .lds-circle, #ays-quiz-container-11 .lds-facebook div, #ays-quiz-container-11 .lds-ellipsis div{ background: #000; } #ays-quiz-container-11 .lds-ripple div{ border-color: #000; } #ays-quiz-container-11 .lds-dual-ring::after, #ays-quiz-container-11 .lds-hourglass::after{ border-color: #000 transparent #000 transparent; } /* Stars */ #ays-quiz-container-11 .ui.rating .icon, #ays-quiz-container-11 .ui.rating .icon:before { } /* Progress bars */ #ays-quiz-container-11 #ays_finish_quiz_11 .ays-progress { border-color: rgba(0,0,0,0.8); } #ays-quiz-container-11 #ays_finish_quiz_11 .ays-progress-bg { background-color: rgba(0,0,0,0.3); } #ays-quiz-container-11 . { background-color: #27AE60; } #ays-quiz-container-11 . { background-color: #000; } #ays-quiz-container-11 .ays-progress-value { color: #000; text-align: center; } #ays-quiz-container-11 .ays-progress-bar { background-color: #27AE60; } #ays-quiz-container-11 .ays-question-counter .ays-live-bar-wrap { direction:ltr !important; } #ays-quiz-container-11 .ays-live-bar-fill{ color: #000; border-bottom: 2px solid rgba(0,0,0,0.8); text-shadow: 0px 0px 5px #fff; } #ays-quiz-container-11 .ays-live-bar-fill.ays-live-fourth, #ays-quiz-container-11 .ays-live-bar-fill.ays-live-third, #ays-quiz-container-11 .ays-live-bar-fill.ays-live-second { text-shadow: unset; } #ays-quiz-container-11 .ays-live-bar-percent{ display:none; } #ays-quiz-container-11 #ays_finish_quiz_11 .ays_average { text-align: center; } /* Music, Sound */ #ays-quiz-container-11 .ays_music_sound { color:rgb(0,0,0); } /* Dropdown questions scroll bar */ #ays-quiz-container-11 blockquote { border-left-color: #000 !important; } /* Quiz Password */ #ays-quiz-container-11 .ays-start-page > input[id^='ays_quiz_password_val_'], #ays-quiz-container-11 .ays-quiz-password-toggle-visibility-box { width: 100%; } /* Question hint */ #ays-quiz-container-11 .ays_question_hint_container .ays_question_hint_text { background-color:#fff; box-shadow: 0 0 15px 3px rgba(0,0,0,0.6); max-width: 270px; } #ays-quiz-container-11 .ays_question_hint_container .ays_question_hint_text p { max-width: unset; } #ays-quiz-container-11 .ays_questions_hint_max_width_class { max-width: 80%; } /* Information form */ #ays-quiz-container-11 .ays-form-title{ color:rgb(0,0,0); } /* Quiz timer */ #ays-quiz-container-11 div.ays-quiz-redirection-timer, #ays-quiz-container-11 div.ays-quiz-timer{ color: #000; text-align: center; } #ays-quiz-container-11 div.ays-quiz-timer.ays-quiz-message-before-timer:before { font-weight: 500; } /* Quiz title / transformation */ #ays-quiz-container-11 .ays-fs-title{ text-transform: uppercase; font-size: 21px; text-align: center; text-shadow: none; } /* Quiz buttons */ #ays-quiz-container-11 .ays_arrow { color:#000000!important; } #ays-quiz-container-11 input#ays-submit, #ays-quiz-container-11 #ays_finish_quiz_11 .action-button, div#ays-quiz-container-11 #ays_finish_quiz_11 .action-button.ays_restart_button, #ays-quiz-container-11 + .ays-quiz-category-selective-main-container .ays-quiz-category-selective-restart-bttn, #ays-quiz-container-11 .ays-quiz-category-selective-submit-bttn { background: none; background-color: #27AE60; color:#000000; font-size: 17px; padding: 10px 20px; border-radius: 3px; height: auto; letter-spacing: 0; box-shadow: unset; } #ays-quiz-container-11 input#ays-submit, #ays-quiz-container-11 #ays_finish_quiz_11 input.action-button, #ays-quiz-container-11 + .ays-quiz-category-selective-main-container .ays-quiz-category-selective-restart-bttn, #ays-quiz-container-11 .ays-quiz-category-selective-submit-bttn { } #ays-quiz-container-11 #ays_finish_quiz_11 .action-button.ays_check_answer { padding: 5px 10px; font-size: 17px !important; } #ays-quiz-container-11 #ays_finish_quiz_11 .action-button.ays_restart_button { white-space: nowrap; padding: 5px 10px; white-space: normal; } #ays-quiz-container-11 input#ays-submit:hover, #ays-quiz-container-11 input#ays-submit:focus, #ays-quiz-container-11 #ays_finish_quiz_11 .action-button:hover, #ays-quiz-container-11 #ays_finish_quiz_11 .action-button:focus, #ays-quiz-container-11 + .ays-quiz-category-selective-main-container .ays-quiz-category-selective-restart-bttn:hover, #ays-quiz-container-11 .ays-quiz-category-selective-submit-bttn:hover { background: none; box-shadow: 0 0 0 2px #000000; background-color: #27AE60; } #ays-quiz-container-11 .ays_restart_button { color: #000000; } #ays-quiz-container-11 .ays_restart_button_p, #ays-quiz-container-11 .ays_buttons_div { justify-content: center; } #ays-quiz-container-11 .ays_finish.action-button{ margin: 10px 5px; } #ays-quiz-container-11 .ays-share-btn.ays-share-btn-branded { color: #fff; display: inline-block; } #ays-quiz-container-11 .ays_quiz_results .ays-field.checked_answer_div.correct_div input:checked+label { background-color: transparent; } /* Question answers */ #ays-quiz-container-11 .ays-field { border-color: #444; border-style: solid; border-width: 1px; box-shadow: none; } /* Answer maximum length of a text field */ #ays-quiz-container-11 .ays_quiz_question_text_message{ color: #000; text-align: left; font-size: 12px; } div#ays-quiz-container-11 div.ays_quiz_question_text_error_message { color: #ff0000; } #ays-quiz-container-11 .ays-quiz-answers .ays-field:hover{ opacity: 1; } #ays-quiz-container-11 #ays_finish_quiz_11 .ays-field { margin-bottom: 10px; } #ays-quiz-container-11 #ays_finish_quiz_11 .ays-field.ays_grid_view_item { width: calc(50% - 5px); } #ays-quiz-container-11 #ays_finish_quiz_11 .ays-field.ays_grid_view_item:nth-child(odd) { margin-right: 5px; } #ays-quiz-container-11 #ays_finish_quiz_11 .ays-field input:checked+label:before { border-color: #27AE60; background: #27AE60; background-clip: content-box; } #ays-quiz-container-11 .ays-quiz-answers div.ays-text-right-answer { color: #000; } /* Questions answer image */ #ays-quiz-container-11 .ays-answer-image { width:50%; } /* Questions answer right/wrong icons */ #ays-quiz-container-11 .ays-field input~label.answered.correct:after{ content: url('https://gogoacademy.net/wp-content/plugins/quiz-maker/public/images/correct.png'); } #ays-quiz-container-11 .ays-field input~label.answered.wrong:after{ content: url('https://gogoacademy.net/wp-content/plugins/quiz-maker/public/images/wrong.png'); } /* Dropdown questions */ #ays-quiz-container-11 #ays_finish_quiz_11 .ays-field .select2-container--default .select2-selection--single { border-bottom: 2px solid #27AE60; background-color: #27AE60; } #ays-quiz-container-11 .ays-field .select2-container--default .select2-selection--single .select2-selection__placeholder, #ays-quiz-container-11 .ays-field .select2-container--default .select2-selection--single .select2-selection__rendered, #ays-quiz-container-11 .ays-field .select2-container--default .select2-selection--single .select2-selection__arrow { color: #ffffff; } #ays-quiz-container-11 .select2-container--default .select2-search--dropdown .select2-search__field:focus, #ays-quiz-container-11 .select2-container--default .select2-search--dropdown .select2-search__field { outline: unset; padding: 0.75rem; } #ays-quiz-container-11 .ays-field .select2-container--default .select2-selection--single .select2-selection__rendered, #ays-quiz-container-11 .select2-container--default .select2-results__option--highlighted[aria-selected] { background-color: #27AE60; } #ays-quiz-container-11 .ays-field .select2-container--default, #ays-quiz-container-11 .ays-field .select2-container--default .selection, #ays-quiz-container-11 .ays-field .select2-container--default .dropdown-wrapper, #ays-quiz-container-11 .ays-field .select2-container--default .select2-selection--single .select2-selection__rendered, #ays-quiz-container-11 .ays-field .select2-container--default .select2-selection--single .select2-selection__rendered .select2-selection__placeholder, #ays-quiz-container-11 .ays-field .select2-container--default .select2-selection--single .select2-selection__arrow, #ays-quiz-container-11 .ays-field .select2-container--default .select2-selection--single .select2-selection__arrow b[role='presentation'] { font-size: 16px !important; } #ays-quiz-container-11 .select2-container--default .select2-results__option { padding: 6px; } /* Dropdown questions scroll bar */ #ays-quiz-container-11 .select2-results__options::-webkit-scrollbar { width: 7px; } #ays-quiz-container-11 .select2-results__options::-webkit-scrollbar-track { background-color: rgba(255,255,255,0.35); } #ays-quiz-container-11 .select2-results__options::-webkit-scrollbar-thumb { transition: .3s ease-in-out; background-color: rgba(0,0,0,0.55); } #ays-quiz-container-11 .select2-results__options::-webkit-scrollbar-thumb:hover { transition: .3s ease-in-out; background-color: rgba(0,0,0,0.85); } /* Audio / Video */ #ays-quiz-container-11 .mejs-container .mejs-time{ box-sizing: unset; } #ays-quiz-container-11 .mejs-container .mejs-time-rail { padding-top: 15px; } #ays-quiz-container-11 .mejs-container .mejs-mediaelement video { margin: 0; } /* Limitation */ #ays-quiz-container-11 .ays-quiz-limitation-count-of-takers { padding: 50px; } #ays-quiz-container-11 div.ays-quiz-results-toggle-block span.ays-show-res-toggle.ays-res-toggle-show, #ays-quiz-container-11 div.ays-quiz-results-toggle-block span.ays-show-res-toggle.ays-res-toggle-hide{ color: #000; } #ays-quiz-container-11 div.ays-quiz-results-toggle-block input:checked + label.ays_switch_toggle { border: 1px solid #000; } #ays-quiz-container-11 div.ays-quiz-results-toggle-block input:checked + label.ays_switch_toggle { border: 1px solid #000; } #ays-quiz-container-11 div.ays-quiz-results-toggle-block input:checked + label.ays_switch_toggle:after{ background: #000; } #ays-quiz-container-11.ays_quiz_elegant_dark div.ays-quiz-results-toggle-block input:checked + label.ays_switch_toggle:after, #ays-quiz-container-11.ays_quiz_rect_dark div.ays-quiz-results-toggle-block input:checked + label.ays_switch_toggle:after{ background: #000; } /* Hestia theme (Version: 3.0.16) | Start */ #ays-quiz-container-11 .mejs-container .mejs-inner .mejs-controls .mejs-button > button:hover, #ays-quiz-container-11 .mejs-container .mejs-inner .mejs-controls .mejs-button > button { box-shadow: unset; background-color: transparent; } #ays-quiz-container-11 .mejs-container .mejs-inner .mejs-controls .mejs-button > button { margin: 10px 6px; } /* Hestia theme (Version: 3.0.16) | End */ /* Go theme (Version: 1.4.3) | Start */ #ays-quiz-container-11 label[for^='ays-answer']:before, #ays-quiz-container-11 label[for^='ays-answer']:before { -webkit-mask-image: unset; mask-image: unset; } #ays-quiz-container-11.ays_quiz_classic_light .ays-field input:checked+label.answered.correct:before, #ays-quiz-container-11.ays_quiz_classic_dark .ays-field input:checked+label.answered.correct:before { background-color: #27AE60 !important; } /* Go theme (Version: 1.4.3) | End */ #ays-quiz-container-11 .ays_quiz_results fieldset.ays_fieldset .ays_quiz_question .wp-video { width: 100% !important; max-width: 100%; } /* Classic Dark / Classic Light */ /* Dropdown questions right/wrong styles */ #ays-quiz-container-11.ays_quiz_classic_dark .correct_div, #ays-quiz-container-11.ays_quiz_classic_light .correct_div{ border-color:green !important; opacity: 1 !important; background-color: rgba(39,174,96,0.4) !important; } #ays-quiz-container-11.ays_quiz_classic_dark .correct_div .selected-field, #ays-quiz-container-11.ays_quiz_classic_light .correct_div .selected-field { padding: 0px 10px 0px 10px; color: green !important; } #ays-quiz-container-11.ays_quiz_classic_dark .wrong_div, #ays-quiz-container-11.ays_quiz_classic_light .wrong_div{ border-color:red !important; opacity: 1 !important; background-color: rgba(243,134,129,0.4) !important; } #ays-quiz-container-11.ays_quiz_classic_dark .ays-field, #ays-quiz-container-11.ays_quiz_classic_light .ays-field { text-align: left; /*margin-bottom: 10px;*/ padding: 0; transition: .3s ease-in-out; } #ays-quiz-container-11 .ays-quiz-close-full-screen { fill: #000; } #ays-quiz-container-11 .ays-quiz-open-full-screen { fill: #000; } #ays-quiz-container-11 .ays_quiz_login_form p{ color: #000; } @media screen and (max-width: 768px){ #ays-quiz-container-11{ max-width: 100%; } #ays-quiz-container-11 .ays_quiz_question p { font-size: 16px; } #ays-quiz-container-11 .select2-container, #ays-quiz-container-11 .ays-field * { font-size: 15px !important; } div#ays-quiz-container-11 input#ays-submit, div#ays-quiz-container-11 #ays_finish_quiz_11 .action-button, div#ays-quiz-container-11 #ays_finish_quiz_11 .action-button.ays_restart_button, #ays-quiz-container-11 + .ays-quiz-category-selective-main-container .ays-quiz-category-selective-restart-bttn, #ays-quiz-container-11 .ays-quiz-category-selective-submit-bttn { font-size: 17px; } /* Quiz title / mobile font size */ div#ays-quiz-container-11 .ays-fs-title { font-size: 21px; } /* Question explanation / mobile font size */ #ays-quiz-container-11 .ays_questtion_explanation p { font-size:16px; } /* Wrong answers / mobile font size */ #ays-quiz-container-11 .wrong_answer_text p { font-size:16px; } /* Right answers / mobile font size */ #ays-quiz-container-11 .right_answer_text p { font-size:16px; } /* Note text / mobile font size */ #ays-quiz-container-11 .ays-quiz-question-note-message-box p { font-size:14px; } } /* Custom css styles */ /* RTL direction styles */ #ays-quiz-container-11 p { margin: 0.625em; } #ays-quiz-container-11 .ays-field.checked_answer_div input:checked+label { background-color: rgba(39,174,96,0.6); } #ays-quiz-container-11.ays_quiz_classic_light .enable_correction .ays-field.checked_answer_div input:checked+label, #ays-quiz-container-11.ays_quiz_classic_dark .enable_correction .ays-field.checked_answer_div input:checked+label { background-color: transparent; } #ays-quiz-container-11 .ays-field.checked_answer_div input:checked+label:hover { background-color: rgba(39,174,96,0.8); } #ays-quiz-container-11 .ays-field:hover label{ background: rgba(39,174,96,0.8); /* border-radius: 4px; */ color: #fff; transition: all .3s; } #ays-quiz-container-11 #ays_finish_quiz_11 .action-button:hover, #ays-quiz-container-11 #ays_finish_quiz_11 .action-button:focus, #ays-quiz-container-11 + .ays-quiz-category-selective-main-container .ays-quiz-category-selective-restart-bttn:hover, #ays-quiz-container-11 .ays-quiz-category-selective-submit-bttn:focus { box-shadow: 0 0 0 2px white, 0 0 0 3px #27AE60; background: #27AE60; } if(typeof aysQuizOptions === 'undefined'){ var aysQuizOptions = []; } aysQuizOptions['11'] = 'eyJxdWl6X3ZlcnNpb24iOiI2LjMuNi44IiwiY29yZV92ZXJzaW9uIjoiNi4wLjMiLCJwaHBfdmVyc2lvbiI6IjcuNC4zMiIsImNvbG9yIjoiIzI3QUU2MCIsImJnX2NvbG9yIjoiI2ZmZiIsInRleHRfY29sb3IiOiIjMDAwIiwiaGVpZ2h0IjozNTAsIndpZHRoIjo0MDAsImVuYWJsZV9sb2dnZWRfdXNlcnMiOiJvZmYiLCJpbmZvcm1hdGlvbl9mb3JtIjoiZGlzYWJsZSIsImZvcm1fbmFtZSI6bnVsbCwiZm9ybV9lbWFpbCI6bnVsbCwiZm9ybV9waG9uZSI6bnVsbCwiaW1hZ2Vfd2lkdGgiOiIiLCJpbWFnZV9oZWlnaHQiOiIiLCJlbmFibGVfY29ycmVjdGlvbiI6Im9mZiIsImVuYWJsZV9wcm9ncmVzc19iYXIiOiJvbiIsImVuYWJsZV9xdWVzdGlvbnNfcmVzdWx0Ijoib24iLCJyYW5kb21pemVfcXVlc3Rpb25zIjoib24iLCJyYW5kb21pemVfYW5zd2VycyI6Im9uIiwiZW5hYmxlX3F1ZXN0aW9uc19jb3VudGVyIjoib24iLCJlbmFibGVfcmVzdHJpY3Rpb25fcGFzcyI6Im9mZiIsInJlc3RyaWN0aW9uX3Bhc3NfbWVzc2FnZSI6IiIsInVzZXJfcm9sZSI6W10sImN1c3RvbV9jc3MiOiIiLCJsaW1pdF91c2VycyI6Im9mZiIsImxpbWl0YXRpb25fbWVzc2FnZSI6IiIsInJlZGlyZWN0X3VybCI6IiIsInJlZGlyZWN0aW9uX2RlbGF5IjowLCJhbnN3ZXJzX3ZpZXciOiJsaXN0IiwiZW5hYmxlX3J0bF9kaXJlY3Rpb24iOiJvZmYiLCJlbmFibGVfbG9nZ2VkX3VzZXJzX21lc3NhZ2UiOiIiLCJxdWVzdGlvbnNfY291bnQiOiIiLCJlbmFibGVfcXVlc3Rpb25fYmFuayI6Im9uIiwiZW5hYmxlX2xpdmVfcHJvZ3Jlc3NfYmFyIjoib24iLCJlbmFibGVfcGVyY2VudF92aWV3Ijoib24iLCJlbmFibGVfYXZlcmFnZV9zdGF0aXN0aWNhbCI6Im9uIiwiZW5hYmxlX25leHRfYnV0dG9uIjoib24iLCJlbmFibGVfcHJldmlvdXNfYnV0dG9uIjoib24iLCJlbmFibGVfYXJyb3dzIjoib2ZmIiwidGltZXJfdGV4dCI6IiIsInF1aXpfdGhlbWUiOiJjbGFzc2ljX2xpZ2h0IiwiZW5hYmxlX3NvY2lhbF9idXR0b25zIjoib24iLCJyZXN1bHRfdGV4dCI6IiIsImVuYWJsZV9wYXNzX2NvdW50Ijoib2ZmIiwiaGlkZV9zY29yZSI6Im9mZiIsInJhdGVfZm9ybV90aXRsZSI6IiIsImJveF9zaGFkb3dfY29sb3IiOiIjMDAwIiwicXVpel9ib3JkZXJfcmFkaXVzIjoiMCIsInF1aXpfYmdfaW1hZ2UiOiIiLCJxdWl6X2JvcmRlcl93aWR0aCI6IjEiLCJxdWl6X2JvcmRlcl9zdHlsZSI6InNvbGlkIiwicXVpel9ib3JkZXJfY29sb3IiOiIjMDAwIiwicXVpel9sb2FkZXIiOiJob3VyZ2xhc3MiLCJjcmVhdGVfZGF0ZSI6IjIwMjMtMDEtMTUgMTg6MDU6MTgiLCJhdXRob3IiOiJ7XCJpZFwiOlwiMVwiLFwibmFtZVwiOlwiZ29nb3JhYmVpXCJ9IiwicXVlc3RfYW5pbWF0aW9uIjoic2hha2UiLCJmb3JtX3RpdGxlIjoiIiwiZW5hYmxlX2JnX211c2ljIjoib2ZmIiwicXVpel9iZ19tdXNpYyI6IiIsImFuc3dlcnNfZm9udF9zaXplIjoxNSwic2hvd19jcmVhdGVfZGF0ZSI6Im9uIiwic2hvd19hdXRob3IiOiJvbiIsImVuYWJsZV9lYXJseV9maW5pc2giOiJvbiIsImFuc3dlcnNfcndfdGV4dHMiOiJvbl9wYXNzaW5nIiwiZGlzYWJsZV9zdG9yZV9kYXRhIjoib2ZmIiwiZW5hYmxlX2JhY2tncm91bmRfZ3JhZGllbnQiOiJvZmYiLCJiYWNrZ3JvdW5kX2dyYWRpZW50X2NvbG9yXzEiOiIjMDAwIiwiYmFja2dyb3VuZF9ncmFkaWVudF9jb2xvcl8yIjoiI2ZmZiIsInF1aXpfZ3JhZGllbnRfZGlyZWN0aW9uIjoidmVydGljYWwiLCJyZWRpcmVjdF9hZnRlcl9zdWJtaXQiOiJvZmYiLCJzdWJtaXRfcmVkaXJlY3RfdXJsIjoiIiwic3VibWl0X3JlZGlyZWN0X2RlbGF5IjoiMCIsInByb2dyZXNzX2Jhcl9zdHlsZSI6InNlY29uZCIsImVuYWJsZV9leGl0X2J1dHRvbiI6Im9mZiIsImV4aXRfcmVkaXJlY3RfdXJsIjoiIiwiaW1hZ2Vfc2l6aW5nIjoiY292ZXIiLCJxdWl6X2JnX2ltYWdlX3Bvc2l0aW9uIjoiY2VudGVyIGNlbnRlciIsImN1c3RvbV9jbGFzcyI6IiIsImVuYWJsZV9zb2NpYWxfbGlua3MiOiJvbiIsInNvY2lhbF9saW5rcyI6eyJsaW5rZWRpbl9saW5rIjoiIiwiZmFjZWJvb2tfbGluayI6IiIsInR3aXR0ZXJfbGluayI6IiIsInZrb250YWt0ZV9saW5rIjoiIiwiaW5zdGFncmFtX2xpbmsiOiIiLCJ5b3V0dWJlX2xpbmsiOiIiLCJiZWhhbmNlX2xpbmsiOiIifSwic2hvd19xdWl6X3RpdGxlIjoib24iLCJzaG93X3F1aXpfZGVzYyI6Im9uIiwic2hvd19sb2dpbl9mb3JtIjoib2ZmIiwibW9iaWxlX21heF93aWR0aCI6IiIsImxpbWl0X3VzZXJzX2J5IjoiaXAiLCJhY3RpdmVfZGF0ZV9jaGVjayI6Im9mZiIsImFjdGl2ZUludGVydmFsIjoiMjAyMy0wMS0xNiAxNTowMTo0MCIsImRlYWN0aXZlSW50ZXJ2YWwiOiIyMDIzLTAxLTE2IDE1OjAxOjQwIiwiYWN0aXZlX2RhdGVfcHJlX3N0YXJ0X21lc3NhZ2UiOiI8cD5UaGUgcXVpeiB3aWxsIGJlIGF2YWlsYWJsZSBzb29uITxcL3A+IiwiYWN0aXZlX2RhdGVfbWVzc2FnZSI6IjxwPlRoZSBxdWl6IGhhcyBleHBpcmVkITxcL3A+IiwiZXhwbGFuYXRpb25fdGltZSI6IjQiLCJlbmFibGVfY2xlYXJfYW5zd2VyIjoib2ZmIiwic2hvd19jYXRlZ29yeSI6Im9uIiwic2hvd19xdWVzdGlvbl9jYXRlZ29yeSI6Im9uIiwiZGlzcGxheV9zY29yZSI6ImJ5X2NvcnJlY3RuZXNzIiwiZW5hYmxlX3J3X2FzbndlcnNfc291bmRzIjoib24iLCJhbnNfcmlnaHRfd3JvbmdfaWNvbiI6ImRlZmF1bHQiLCJxdWl6X2JnX2ltZ19pbl9maW5pc2hfcGFnZSI6Im9mZiIsImZpbmlzaF9hZnRlcl93cm9uZ19hbnN3ZXIiOiJvZmYiLCJhZnRlcl90aW1lcl90ZXh0IjoiIiwiZW5hYmxlX2VudGVyX2tleSI6Im9uIiwiYnV0dG9uc190ZXh0X2NvbG9yIjoiIzAwMDAwMCIsImJ1dHRvbnNfcG9zaXRpb24iOiJjZW50ZXIiLCJzaG93X3F1ZXN0aW9uc19leHBsYW5hdGlvbiI6Im9uX3Jlc3VsdHNfcGFnZSIsImVuYWJsZV9hdWRpb19hdXRvcGxheSI6Im9mZiIsImJ1dHRvbnNfc2l6ZSI6Im1lZGl1bSIsImJ1dHRvbnNfZm9udF9zaXplIjoiMTciLCJidXR0b25zX3dpZHRoIjoiIiwiYnV0dG9uc19sZWZ0X3JpZ2h0X3BhZGRpbmciOiIyMCIsImJ1dHRvbnNfdG9wX2JvdHRvbV9wYWRkaW5nIjoiMTAiLCJidXR0b25zX2JvcmRlcl9yYWRpdXMiOiIzIiwiZW5hYmxlX2xlYXZlX3BhZ2UiOiJvbiIsImVuYWJsZV90YWNrZXJzX2NvdW50Ijoib2ZmIiwidGFja2Vyc19jb3VudCI6IiIsInBhc3Nfc2NvcmUiOjcwLCJwYXNzX3Njb3JlX21lc3NhZ2UiOiI8aDQgc3R5bGU9XCJ0ZXh0LWFsaWduOiBjZW50ZXJcIj5Db25ncmF0dWxhdGlvbnMhPFwvaDQ+XHJcbjxwIHN0eWxlPVwidGV4dC1hbGlnbjogY2VudGVyXCI+WW91IHBhc3NlZCB0aGUgcXVpeiE8XC9wPiIsImZhaWxfc2NvcmVfbWVzc2FnZSI6IjxoNCBzdHlsZT1cInRleHQtYWxpZ246IGNlbnRlclwiPk9vcHMhPFwvaDQ+XHJcbjxwIHN0eWxlPVwidGV4dC1hbGlnbjogY2VudGVyXCI+WW91IGhhdmUgbm90IHBhc3NlZCB0aGUgcXVpeiEgPGJyIFwvPlxyXG5UcnkgYWdhaW4hPFwvcD4iLCJxdWVzdGlvbl9mb250X3NpemUiOjE2LCJxdWl6X3dpZHRoX2J5X3BlcmNlbnRhZ2VfcHgiOiJwaXhlbHMiLCJxdWVzdGlvbnNfaGludF9pY29uX29yX3RleHQiOiJkZWZhdWx0IiwicXVlc3Rpb25zX2hpbnRfdmFsdWUiOiIiLCJlbmFibGVfZWFybHlfZmluc2hfY29tZmlybV9ib3giOiJvbiIsImVuYWJsZV9xdWVzdGlvbnNfb3JkZXJpbmdfYnlfY2F0Ijoib24iLCJzaG93X3NjaGVkdWxlX3RpbWVyIjoib2ZmIiwic2hvd190aW1lcl90eXBlIjoiY291bnRkb3duIiwicXVpel9sb2FkZXJfdGV4dF92YWx1ZSI6IiIsImhpZGVfY29ycmVjdF9hbnN3ZXJzIjoib2ZmIiwic2hvd19pbmZvcm1hdGlvbl9mb3JtIjoib2ZmIiwicXVpel9sb2FkZXJfY3VzdG9tX2dpZiI6IiIsImRpc2FibGVfaG92ZXJfZWZmZWN0Ijoib2ZmIiwicXVpel9sb2FkZXJfY3VzdG9tX2dpZl93aWR0aCI6MTAwLCJwcm9ncmVzc19saXZlX2Jhcl9zdHlsZSI6ImRlZmF1bHQiLCJxdWl6X3RpdGxlX3RyYW5zZm9ybWF0aW9uIjoidXBwZXJjYXNlIiwic2hvd19hbnN3ZXJzX251bWJlcmluZyI6Im5vbmUiLCJxdWl6X2ltYWdlX3dpZHRoX2J5X3BlcmNlbnRhZ2VfcHgiOiJwaXhlbHMiLCJxdWl6X2ltYWdlX2hlaWdodCI6IiIsInF1aXpfYmdfaW1nX29uX3N0YXJ0X3BhZ2UiOiJvZmYiLCJxdWl6X2JveF9zaGFkb3dfeF9vZmZzZXQiOjAsInF1aXpfYm94X3NoYWRvd195X29mZnNldCI6MCwicXVpel9ib3hfc2hhZG93X3pfb2Zmc2V0IjoxNSwicXVpel9xdWVzdGlvbl90ZXh0X2FsaWdubWVudCI6ImNlbnRlciIsInF1aXpfYXJyb3dfdHlwZSI6ImRlZmF1bHQiLCJxdWl6X3Nob3dfd3JvbmdfYW5zd2Vyc19maXJzdCI6Im9mZiIsInF1aXpfZGlzcGxheV9hbGxfcXVlc3Rpb25zIjoib2ZmIiwicXVpel90aW1lcl9yZWRfd2FybmluZyI6Im9mZiIsInF1aXpfc2NoZWR1bGVfdGltZXpvbmUiOiJVVEMrMCIsInF1ZXN0aW9uc19oaW50X2J1dHRvbl92YWx1ZSI6IiIsInF1aXpfdGFja2Vyc19tZXNzYWdlIjoiPHA+VGhpcyBxdWl6IGlzIGV4cGlyZWQhPFwvcD4iLCJxdWl6X2VuYWJsZV9saW5rZWRpbl9zaGFyZV9idXR0b24iOiJvbiIsInF1aXpfZW5hYmxlX2ZhY2Vib29rX3NoYXJlX2J1dHRvbiI6Im9uIiwicXVpel9lbmFibGVfdHdpdHRlcl9zaGFyZV9idXR0b24iOiJvbiIsInF1aXpfbWFrZV9yZXNwb25zZXNfYW5vbnltb3VzIjoib2ZmIiwicXVpel9tYWtlX2FsbF9yZXZpZXdfbGluayI6Im9mZiIsInNob3dfcXVlc3Rpb25zX251bWJlcmluZyI6Im5vbmUiLCJxdWl6X21lc3NhZ2VfYmVmb3JlX3RpbWVyIjoiIiwiZW5hYmxlX3Bhc3N3b3JkIjoib2ZmIiwicGFzc3dvcmRfcXVpeiI6IiIsInF1aXpfcGFzc3dvcmRfbWVzc2FnZSI6IiIsImVuYWJsZV9zZWVfcmVzdWx0X2NvbmZpcm1fYm94Ijoib24iLCJkaXNwbGF5X2ZpZWxkc19sYWJlbHMiOiJvZmYiLCJlbmFibGVfZnVsbF9zY3JlZW5fbW9kZSI6Im9mZiIsInF1aXpfZW5hYmxlX3Bhc3N3b3JkX3Zpc2liaWxpdHkiOiJvZmYiLCJxdWVzdGlvbl9tb2JpbGVfZm9udF9zaXplIjoxNiwiYW5zd2Vyc19tb2JpbGVfZm9udF9zaXplIjoxNSwic29jaWFsX2J1dHRvbnNfaGVhZGluZyI6IiIsInF1aXpfZW5hYmxlX3Zrb250YWt0ZV9zaGFyZV9idXR0b24iOiJvbiIsImFuc3dlcnNfYm9yZGVyIjoib24iLCJhbnN3ZXJzX2JvcmRlcl93aWR0aCI6MSwiYW5zd2Vyc19ib3JkZXJfc3R5bGUiOiJzb2xpZCIsImFuc3dlcnNfYm9yZGVyX2NvbG9yIjoiIzQ0NCIsInNvY2lhbF9saW5rc19oZWFkaW5nIjoiIiwicXVpel9lbmFibGVfcXVlc3Rpb25fY2F0ZWdvcnlfZGVzY3JpcHRpb24iOiJvbiIsImFuc3dlcnNfbWFyZ2luIjoxMCwicXVpel9tZXNzYWdlX2JlZm9yZV9yZWRpcmVjdF90aW1lciI6IiIsImJ1dHRvbnNfbW9iaWxlX2ZvbnRfc2l6ZSI6MTcsImFuc3dlcnNfYm94X3NoYWRvdyI6Im9mZiIsImFuc3dlcnNfYm94X3NoYWRvd19jb2xvciI6IiMwMDAiLCJxdWl6X2Fuc3dlcl9ib3hfc2hhZG93X3hfb2Zmc2V0IjowLCJxdWl6X2Fuc3dlcl9ib3hfc2hhZG93X3lfb2Zmc2V0IjowLCJxdWl6X2Fuc3dlcl9ib3hfc2hhZG93X3pfb2Zmc2V0IjoxMCwicXVpel9jcmVhdGVfYXV0aG9yIjoxLCJxdWl6X2VuYWJsZV90aXRsZV90ZXh0X3NoYWRvdyI6Im9mZiIsInF1aXpfdGl0bGVfdGV4dF9zaGFkb3dfY29sb3IiOiIjMzMzIiwicXVpel90aXRsZV90ZXh0X3NoYWRvd194X29mZnNldCI6MiwicXVpel90aXRsZV90ZXh0X3NoYWRvd195X29mZnNldCI6MiwicXVpel90aXRsZV90ZXh0X3NoYWRvd196X29mZnNldCI6MiwicXVpel9zaG93X29ubHlfd3JvbmdfYW5zd2VycyI6Im9uIiwicXVpel90aXRsZV9mb250X3NpemUiOjIxLCJxdWl6X3RpdGxlX21vYmlsZV9mb250X3NpemUiOjIxLCJxdWl6X3Bhc3N3b3JkX3dpZHRoIjoiIiwicXVpel9yZXZpZXdfcGxhY2Vob2xkZXJfdGV4dCI6IiIsInF1aXpfbWFrZV9yZXZpZXdfcmVxdWlyZWQiOiJvZmYiLCJxdWl6X2VuYWJsZV9yZXN1bHRzX3RvZ2dsZSI6Im9mZiIsInJlcXVpcmVkX2ZpZWxkcyI6bnVsbCwiZW5hYmxlX3RpbWVyIjoib2ZmIiwiZW5hYmxlX3F1aXpfcmF0ZSI6Im9mZiIsImVuYWJsZV9yYXRlX2F2ZyI6Im9mZiIsImVuYWJsZV9ib3hfc2hhZG93Ijoib24iLCJlbmFibGVfYm9yZGVyIjoib2ZmIiwicXVpel90aW1lcl9pbl90aXRsZSI6Im9mZiIsImVuYWJsZV9yYXRlX2NvbW1lbnRzIjoib2ZmIiwiZW5hYmxlX3Jlc3RhcnRfYnV0dG9uIjoib24iLCJhdXRvZmlsbF91c2VyX2RhdGEiOiJvZmYiLCJ0aW1lciI6MTAwLCJxdWl6X3dhaXRpbmdfdGltZSI6Im9mZiIsInF1aXpfZW5hYmxlX3F1aXpfY2F0ZWdvcnlfZGVzY3JpcHRpb24iOiJvZmYiLCJxdWl6X2VuYWJsZV91c2VyX2NcdTA1NzBvb3NpbmdfYW5vbnltb3VzX2Fzc2Vzc21lbnQiOiJvZmYiLCJzdWJtaXRfcmVkaXJlY3RfYWZ0ZXIiOiIiLCJyd19hbnN3ZXJzX3NvdW5kcyI6ZmFsc2UsImlkIjoiMTEiLCJ0aXRsZSI6Im9yZ2FuaWMgY29tcG91bmRzIGFuZCBjbGFzc2lmaWNhdGlvbiBvZiBIeWRyb2NhcmJvbnMiLCJkZXNjcmlwdGlvbiI6IiIsInF1aXpfaW1hZ2UiOiIiLCJxdWl6X2NhdGVnb3J5X2lkIjoiMyIsInF1ZXN0aW9uX2lkcyI6IjE4MiwxODEsMTgzLDE4NCwxODUsMTg2LDE4NywxODgsMTg5LDE5MCwxOTEsMTkyLDE5MywxOTQsMTk1LDE5NiwxOTcsMTk4LDE5OSwyMDAsMjAxLDIwMiwxODAsMjA3LDIwNiwyMDUsMjA0LDIwMywyMDgsMjMwLDIyOSwyMjgsMjI3LDIyNiwyMjUsMjI0LDIyMywyMjIsMjIxLDIyMCwyMTksMjE4LDIxNywyMTYsMjE1LDIxNCwyMTMsMjEyLDIxMSwyMTAsMjA5Iiwib3JkZXJpbmciOiIxMSIsInB1Ymxpc2hlZCI6IjEiLCJpbnRlcnZhbHMiOm51bGwsInF1aXpfdXJsIjpudWxsLCJxdWl6X2FuaW1hdGlvbl90b3AiOjEwMCwicXVpel9lbmFibGVfYW5pbWF0aW9uX3RvcCI6Im9uIn0=';