Atomic emission spectra, Bohr’s atomic model and modern atomic theory
Atomic emission spectra, Bohr’s atomic model and modern atomic theory
Explore the fascinating world of atomic structure and quantum mechanics. Learn about atomic models, electron behavior, quantum principles, and the wave mechanical theory of the atom. Get insights into orbitals, quantum numbers, and the Heisenberg Uncertainty Principle. Discover the advantages and inadequacies of atomic models and gain an understanding of the modern atomic theory.
Atomic emission spectra, also known as line spectra or atomic line emission spectra, are patterns of distinct, discrete lines of light that are emitted by atoms when they transition from excited states to lower energy states.
These spectra are essential in understanding the electronic structure and behavior of atoms.
Here are the key points to know about atomic emission spectra:
Energy Levels:
- In atoms, electrons occupy different energy levels or shells.
- These energy levels are quantized, meaning they have specific, discrete energy values.
Excitation:
- Atoms can be excited when they absorb energy, often in the form of photons (light).
- This excitation causes electrons to move to higher energy levels.
De-Excitation:
- When excited electrons return to lower energy levels, they release the excess energy in the form of photons, which can be observed as light.
- The emitted light has discrete wavelengths and energies, which correspond to the energy differences between the excited and lower energy levels.
Line Spectra:
- Atomic emission spectra consist of lines of specific wavelengths, often visible in the form of colored lines on a spectrograph.
- Each line corresponds to a specific electronic transition between energy levels.
Unique for Each Element:
- Each element has a unique atomic emission spectrum.
- The patterns of lines are like a fingerprint for that element.
- This is the basis for techniques like spectroscopy, which is used for elemental identification.
The line spectrum of hydrogen atom
- The line spectrum of a hydrogen atom is one of the most well-known examples of an atomic emission spectrum.
- It consists of a series of distinct, discrete lines of light that are emitted when the electrons in a hydrogen atom transition between different energy levels.

|
Color |
Violet |
blue |
Green |
red |
|
wavelength |
410 nm |
434 nm |
486 nm |
656 nm |
- The line spectrum of a hydrogen atom is critical in the study of atomic structure and has played a significant role in the development of quantum mechanics.
Here are some key features of the line spectrum of a hydrogen atom:
Balmer Series:
The most famous and prominent part of the hydrogen line spectrum is the Balmer series.
This series represents transitions of electrons in hydrogen from higher energy levels to the second energy level (n = 2).
The lines in the Balmer series fall in the visible region of the electromagnetic spectrum.
The most significant lines in this series are Hα, Hβ, Hγ, and Hδ, corresponding to transitions from n = 3, 4, 5, and 6 to n = 2, respectively.
Lyman and Paschen Series:
Hydrogen also has other series of lines in its spectrum.
The Lyman series represents transitions of electrons to the first energy level (n = 1), and the lines in this series fall in the ultraviolet region.
The Paschen series represents transitions to the third energy level (n = 3), and the lines in this series fall in the infrared region.
Energy Levels:
The lines in the hydrogen spectrum are a result of the quantized energy levels in the atom.
As electrons move between these energy levels, they emit or absorb photons with specific energies corresponding to the energy difference between the levels.
Wavelengths and Energies:
Each line in the hydrogen spectrum corresponds to a specific wavelength of light and energy.

Bohr s Atomic model
Bohr’s atomic model, proposed by Danish physicist Niels Bohr in 1913, was a significant advancement in our understanding of atomic structure and played a crucial role in the development of quantum mechanics. This model introduced the concept of quantized energy levels and successfully explained the line spectrum of hydrogen.
Here are the key features and principles of Bohr’s atomic model:
Bohr’s model postulated that
Quantization of Energy Levels:
- Electrons in an atom can only exist in specific, quantized energy levels or orbits.
- These energy levels are also referred to as electron shells.
- Electrons can transition between these levels by absorbing or emitting energy in the form of photons.
Electron Orbits:
- Electrons move in circular orbits around the nucleus.
- Each orbit is associated with a specific energy level.
- The closer an electron is to the nucleus, the lower its energy level.

Fixed Angular Momentum:
- Bohr suggested that electrons have fixed angular momentum in their orbits.
- This means that they move in stable, well-defined paths without emitting radiation, which was a problem in the classical Rutherford model.
Radiation Emission:
- When an electron transitions from a higher energy level to a lower one, it emits a photon of energy.
- The energy of the emitted photon is equal to the energy difference between the two energy levels, and its frequency and wavelength can be calculated using Planck’s constant and the speed of light.

Stability of Certain Orbits:
- Bohr’s model specified that certain orbits are more stable than others. Electrons in the lowest energy level (n = 1) are in the most stable orbit, and transitions to this orbit result in the emission of photons in the ultraviolet range, forming the Lyman series for hydrogen.
Hydrogen Spectrum:
One of the most significant achievements of Bohr’s model was its ability to explain the line spectrum of hydrogen, including the Balmer, Lyman, and Paschen series.
These series represent transitions of electrons in hydrogen between different energy levels.
Limitations of Bohr’s model:
Bohr’s model was successful in explaining the hydrogen atom but had limitations when applied to atoms with more than one electron.
It did not fully address the complex interactions between electrons and the nucleus.
-It can explain spectrum of any atom than Hydrogen
Bohr s Postulates :points that agree with Rutherford postulates
Niels Bohr’s atomic model was developed as an improvement upon Ernest Rutherford’s atomic model, which had certain limitations.
While Bohr introduced new concepts and postulates to address these limitations, his model still retained some key ideas from Rutherford’s model.
Here are the postulates of Bohr’s model that align with Rutherford’s postulates:
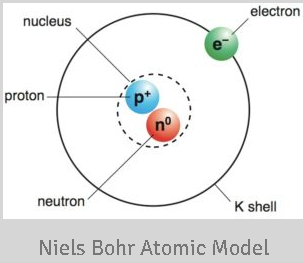
Central Nucleus:
Both Rutherford’s and Bohr’s models agree on the presence of a central, positively charged nucleus at the center of the atom.
Rutherford’s model was the first to propose that most of the atom’s mass is concentrated in the nucleus.
Electron Orbits:
- Bohr’s model retains the concept of electrons orbiting the nucleus, similar to Rutherford’s model.
- However, Bohr’s model introduces quantized energy levels for these orbits, in contrast to the continuous orbits suggested by Rutherford.
Electron Mass and Charge:
Both models acknowledge that electrons have mass and a negative charge.
Rutherford’s model was among the first to establish the existence of electrons in the atomic structure.
Electron-Nucleus Attraction:
Rutherford’s model proposed that electrons are attracted to the positively charged nucleus due to electrostatic forces.
Bohr’s model maintains this concept of electrostatic attraction, but it adds the idea that electrons can only exist in certain stable orbits.
Emission of Radiation:
Rutherford’s model suggested that as electrons move around the nucleus, they should emit radiation.
Bohr’s model addresses this issue by postulating that electrons in stable orbits do not continuously emit radiation.
They only emit or absorb energy when transitioning between energy levels.
It’s important to note that while Bohr’s model builds upon Rutherford’s ideas and addresses some of its shortcomings, it introduces new and fundamental concepts related to quantization of energy levels, stability of orbits, and the explanation of line spectra that were not part of Rutherford’s model.
Therefore, Bohr’s model represents a significant advancement in our understanding of atomic structure, but it also diverges from Rutherford’s model in important ways.
Bohr s Postulates :new points
Niels Bohr’s atomic model introduced several new postulates and concepts that were not part of Ernest Rutherford’s earlier atomic model. These new postulates were crucial in explaining the line spectra of hydrogen and the quantization of energy levels in atoms.
Here are the key new postulates in Bohr’s atomic model:
Quantized Energy Levels:
- Bohr proposed that electrons in an atom can only occupy certain specific energy levels or orbits.
- These energy levels are quantized, meaning they have discrete and well-defined energy values.
- Electrons are not allowed to exist in intermediate energy levels between these quantized states.
Angular Momentum Quantization:
- Bohr introduced the concept of quantized angular momentum for electrons in his model.
- The angular momentum of an electron in a particular orbit is an integer multiple of Planck’s constant divided by 2π (h/2π).
- This quantization of angular momentum helps to determine the allowed energy levels and radii of the electron orbits.
Stable Orbits:
- According to Bohr’s model, certain electron orbits are more stable than others.
- Electrons in the lowest energy level (n = 1) are in the most stable orbit.
- Electrons in stable orbits do not emit radiation, and transitions to these orbits result in the emission or absorption of energy in the form of photons.
Radiation upon Transition:
- When an electron transitions from a higher energy level to a lower one, it emits a photon with energy equal to the energy difference between the levels.
- The frequency and wavelength of the emitted photon can be calculated using Planck’s constant and the speed of light.
Hydrogen Spectrum Explanation:
- Bohr’s model successfully explained the line spectrum of hydrogen, including the Balmer, Lyman, and Paschen series.
- These series represent transitions of electrons in hydrogen between different energy levels.
- The quantization of energy levels in his model accounted for the discrete and specific wavelengths of the spectral lines.
Energy Levels Correspond to Spectral Lines:
Each spectral line in an atomic emission spectrum corresponds to a specific energy level transition in the atom.
The energy levels are directly related to the observed spectral lines, making it possible to predict and explain the wavelengths of the lines.
However, it’s important to note that Bohr’s model was a transitional step in the development of quantum mechanics, and it was later superseded by more comprehensive quantum models that offered a more complete understanding of atomic structure.
Excited atom
An excited atom is an atom in a higher energy state than its ground state. It occurs when the atom absorbs energy, typically in the form of photons or heat, and as a result, its electrons move to higher energy levels.
When the electrons in an atom transition to higher energy levels, they are considered to be in excited states.

The advantages of Bohr s model
Bohr’s atomic model, while no longer the most complete description of atomic structure, provided several advantages and contributions to the understanding of the atom when it was proposed.
These advantages included:
Explanation of Hydrogen Spectrum:
Bohr’s model successfully explained the line spectrum of hydrogen, including the Balmer, Lyman, and Paschen series.
This was a significant achievement because it provided a quantized model for atomic energy levels that could account for the observed discrete wavelengths of spectral lines.
Quantization of Energy Levels:
Bohr introduced the concept of quantized energy levels for electrons in atoms. This idea laid the groundwork for the development of quantum mechanics and was a significant departure from classical physics. It provided a framework for understanding the discrete nature of atomic energy levels and the behavior of electrons.
Stable Orbits:
- Bohr’s model introduced the idea that certain electron orbits are more stable than others. E
- Electrons in stable orbits do not emit radiation, addressing a problem in Rutherford’s model.
- This concept helped explain why electrons didn’t spiral into the nucleus, as classical electromagnetic theory suggested.
Inadequacies of Bohr s model
Here are some of the main inadequacies of Bohr’s model:
- Limited Applicability: Bohr’s model was primarily developed for hydrogen-like atoms, which have one electron.
- It was not able to accurately describe the behavior of atoms with more than one electron, such as helium, which exhibits complex electron-electron interactions.
- Failure to Explain Fine Structure:
Bohr’s model did not account for the fine structure of spectral lines observed in more detailed experiments.
- Lack of Explanation for Electron Spin:
- Uncertainty Principle:
Bohr’s model did not take into account Heisenberg’s Uncertainty Principle, a fundamental concept in quantum mechanics.
This principle dictates that it is impossible to precisely know both the position and momentum of a particle, which has significant implications for electron behavior.
- Wave-Particle Duality:
Bohr’s model treated electrons solely as particles following classical orbits, ignoring their wave-like behavior, as described by the Schrödinger equation in quantum mechanics.
In summary, Bohr’s model made significant contributions to the understanding of atomic structure and energy quantization. However, its inadequacies and limitations became apparent when applied to more complex atoms and a broader range of phenomena. It was replaced by quantum mechanics, which provided a more complete and accurate framework for describing the behavior of electrons in atoms and molecules.
The principle of modern atomic theory (modification of Bor s model)
Modern atomic theory, often referred to as quantum atomic theory, is an evolution of Niels Bohr’s atomic model.
It incorporates the principles of quantum mechanics and provides a more comprehensive and accurate description of atomic and molecular structure and behavior.
Here are the key principles of modern atomic theory, which can be seen as modifications and extensions of Bohr’s model:
Wave-Particle Duality:
The dual nature of electron
- The particles, including electrons, exhibit both particle-like and wave-like properties.
- This is known as wave-particle duality.
- Electrons are described by wave functions that give the probability distribution of their positions.
- Electrons are particles with mass and charge, and they interact with other particles through classical electrostatic forces.
Particle-Like Nature:
- Electrons are particles with mass and charge, and they interact with other particles through classical electrostatic forces.
- They can be detected as discrete points in experiments, much like classical particles.
Wave-Like Nature:
- Electrons also exhibit wave-like properties, as described by their wave functions in quantum mechanics.
- The wave function represents the probability distribution of finding an electron at a particular location in space.
Heisenberg Uncertainty Principle:
Bohr postulated that: ”It is possible to determine both of the location and velocity of the electron precisely at the same time but by applying the principles of quantum mechanics
The Heisenberg concluded that:
“It is impossible to determine both of the velocity and position of an electron at the same time practically but can use the term of “probability”
because the electron wave motion doesn’t have a certain location
Electron Orbitals:
- In modern atomic theory, electrons are described as occupying three–dimensional regions of space known as orbitals.
- These orbitals represent the probability distribution of finding an electron at a particular location.
- The most common types of orbitals are s, p, d, and f orbitals.
Electron Probability Clouds:
Modern atomic theory provides detailed electron probability density maps, showing the regions where electrons are most likely to be found. These probability clouds are often depicted as electron density plots.
This dual nature is essential for understanding the behavior of subatomic particles, such as electrons.
The wave mechanical theory of the atom
The wave mechanical theory of the atom, also known as quantum mechanics or quantum physics, is a fundamental theory that describes the behavior of particles, particularly electrons, at the atomic and subatomic level.
The Austrian scientist Schrödinger (1926) applied the idea of Plank, Einstein, De Broglie and Heisenberg to establish :
- The wave-mechanical theory of the atom
- Derive a wave equation that could describe the electron wave motion in the atom
Here are the key principles and aspects of the wave mechanical theory of the atom:
These wave functions are solutions to the Schrödinger equation, a fundamental equation of quantum mechanics.
Quantization of Energy Levels: Determine the allowed energy levels
- Quantum mechanics quantizes the energy levels of particles in an atom. Electrons are described as existing in specific energy levels, also known as electron shells or orbitals, each characterized by distinct energy values.
Uncertainty Principle:
Wave functions describe the probability distribution of finding a particle at a particular position and time.
Instead of the fixed circular orbits of electron the concept of electron cloud is used to express the region of space around the nucleus.

on solving Schrodinger s equation it is possible to :
Some of the key possibilities and results that arise from solving the Schrödinger equation include:
Energy Levels:
- The Schrödinger equation yields a set of allowed energy levels for the system.
- These energy levels are quantized, meaning they take on discrete, specific values, and they correspond to the possible energy states of the particles within the system.
Orbitals:
- The wave functions derived from the Schrödinger equation describe electron orbitals.
- These orbitals represent regions of space where electrons are likely to be found.
Electron cloud
It is the region of space around the nucleus in which the electron probability exists in all directions and distances (dimention)
Summary:
This lesson delves into the intriguing world of atomic structure and quantum mechanics. It covers the historical development of atomic models, from Rutherford’s to Bohr’s, and introduces the wave mechanical theory of the atom. You’ll gain insights into orbitals, quantum numbers, and the Heisenberg Uncertainty Principle. Explore the advantages and inadequacies of atomic models and understand the principles of modern atomic theory. This comprehensive guide provides a solid foundation for understanding the behavior of subatomic particles and the intricate quantum world.
Atomic Structure and Quantum Mechanics Quiz:
1-Who proposed the first atomic model that featured electrons orbiting the nucleus like planets?
- a) Niels Bohr
- b) Ernest Rutherford
- c) Albert Einstein
- d) J.J. Thomson
2-In the Bohr model of the atom, what does the principal quantum number (n) represent?
- a) The energy of the electron
- b) The shape of the orbital
- c) The orientation of the orbital
- d) The intrinsic spin of the electron
3-What is the Heisenberg Uncertainty Principle?
- a) The principle that electrons must occupy different energy levels
- b) The principle that electrons repel each other in the same orbital
- c) The principle that there is a fundamental limit to the precision of simultaneously knowing certain properties of a particle
- d) The principle that electrons have both wave-like and particle-like properties
4-What are quantum numbers used to describe in atomic physics?
- a) The charge of the nucleus
- b) The speed of electrons
- c) The behavior of electrons in an atom
- d) The shape of the atomic nucleus
5-Which type of orbital has a spherical shape?
- a) p-orbital
- b) s-orbital
- c) d-orbital
- d) f-orbital
6-True or False: In the Schrödinger equation, wave functions represent the probability distribution of finding a particle at a particular location.
7-How many electrons can occupy a single orbital?
- a) One electron
- b) Two electrons with the same spin
- c) Two electrons with opposite spins
- d) Four electrons
8-Which of the following describes the relationship between electrons and chemical bonding?
- a) Electrons are not involved in chemical bonding.
- b) Electrons in different orbitals do not participate in bonding.
- c) Electrons in orbitals overlap with those of other atoms to form chemical bonds.
- d) Electrons are completely transferred between atoms in a bond.
Answers:
1)b) Ernest Rutherford
2)a) The energy of the electron
3)c) The principle that there is a fundamental limit to the precision of simultaneously knowing certain properties of a particle
4)c) The behavior of electrons in an atom
5)b) s-orbital
6)True
7)b) Two electrons with opposite spins
8)c) Electrons in orbitals overlap with those of other atoms to form chemical bonds.
1-What is the name of the mathematical equation that describes the behavior of particles, including electrons, in quantum mechanics?
- a) Planck’s equation
- b) Bohr’s equation
- c) Schrödinger equation
- d) Heisenberg equation
2-Which of the following quantum numbers is not limited to integer values?
- a) Principal quantum number (n)
- b) Azimuthal quantum number (l)
- c) Magnetic quantum number (m)
- d) Spin quantum number (s)
3-What does the Pauli Exclusion Principle state?
- a) Electrons in the same atom must have the same set of quantum numbers.
- b) Electrons in the same orbital must have the same spin.
- c) No two electrons in the same atom can have the same set of quantum numbers.
- d) Electrons in the same atom must occupy the same energy level.
4-Which of the following elements has the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁴?
- a) Carbon (C)
- b) Neon (Ne)
- c) Silicon (Si)
- d) Sulfur (S)
5-What does the term “ground state” refer to in atomic physics?
- a) The lowest energy state of an atom with all electrons in their lowest energy levels.
- b) The state of an atom that is about to undergo a chemical reaction.
- c) The state of an atom with all electrons in the same orbital.
- d) The state of an atom with no electrons.
Answers:
- c) Schrödinger equation
2) Azimuthal quantum number (l)
3) No two electrons in the same atom can have the same set of quantum numbers.
4) Silicon (Si)
5) The lowest energy state of an atom with all electrons in their lowest energy levels.
REFERENCES
- “Principles of Quantum Mechanics” by R. Shankar – This book provides a comprehensive introduction to the principles of quantum mechanics.
- “Physical Chemistry” by Peter Atkins and Julio de Paula – This textbook covers a wide range of topics in physical chemistry, including quantum mechanics and atomic structure.
- “Chemistry: The Central Science” by Theodore E. Brown, H. Eugene LeMay, and Bruce E. Bursten – This widely used chemistry textbook covers the principles of atomic structure and quantum mechanics.
- “Modern Quantum Mechanics” by J. J. Sakurai and Jim Napolitano – This book is a more advanced text on quantum mechanics and provides a detailed understanding of the subject.
