Ionization potential, electron affinity and electronegativity
Explore the concepts of electron affinity, electronegativity, and ionization potential in chemistry. Learn about their definitions, trends across the periodic table, and the significance of these properties in understanding chemical behavior.
Ionization potential, electron affinity and electronegativity
- Ionization potential, also known as ionization energy.
- It is the amount of energy required to remove an electron from an atom or a positive ion.
- It is typically measured in electron volts (eV) or kilojoules per mole (kJ/mol).
- The process of removing an electron from an atom involves overcoming the attractive forces between the negatively charged electron and the positively charged nucleus.
- ∆H of Ionization has a positive sign because the ionization energy is absorbed energy
- Generally, electrons in inner shells are more tightly bound to the nucleus and require more energy to be removed than electrons in outer shells.
The first ionization potential
It is the energy required to remove the outermost (or highest energy) electron from a neutral atom in its gaseous state.
- Elements with low ionization potentials tend to readily lose electrons and form positive ions.
- Elements with high ionization potentials are less likely to lose electrons and more likely to gain electrons to form negative ions.
- This process can be represented by the equation:
X(g)→X+(g)+ e −
- where X represents the element.
Na(g) + Energy → Na+(g) + e– , H=+496 Kj/mol
Example
The ionization potential of lithium (Li), which is an alkali metal and is located in Group 1 of the periodic table.
The electron configuration of lithium is 1s² 2s¹.
The first ionization potential of lithium is the energy required to remove the outermost electron, which is in the 2s1 orbital.
The process can be represented as follows:
Li(g)→Li+(g)+e− 5.39 eV or 520 kJ/mol
The ionization energy for this process is approximately :5.39 eV or 520 kJ/mol
This means that it takes about 5.39 electron volts of energy to remove one mole of electrons from one mole of lithium atoms to produce one mole of lithium ions (Li+).
Ionization potential of Na
Ii depends on which electron is being removed.
- Sodium has an electron configuration of :
- 1s2 2s 2 2p6 3s1
- so it has one electron in its outermost shell (in the 3s orbital).
- The first ionization potential of sodium is the energy required to remove this outermost electron:
Na(g)→Na+(g)+ e−
The ionization energy for this process is approximately :
5.14 eV or 49 kJ/mol
This means that it takes about 495 kJ/mol of energy to remove one mole of electrons from one mole of sodium atoms to produce one mole of sodium ions (Na+ ).
The relatively low first ionization potential of sodium is characteristic of alkali metals, which generally have low ionization energies due to their configuration with a single electron in their outermost shell.
Second ionization potential
- It is the energy required to remove a second electron from an Ion.
- It is higher than the first ionization potential because, after the removal of the first electron, the remaining electrons experience a stronger effective nuclear charge due to the reduced electron shielding.
For sodium (Na), the process of removing a second electron can be represented as follows:
Na+(g)→Na 2+(g)+ e −
The second ionization potential for sodium is higher than the first but lower than the third, as removing electrons becomes more difficult due to the increased positive charge on the ion.
Third ionization potential
- It is the energy required to remove a third electron from an atom after the first two electrons have already been removed.
- In the case of sodium (Na), the process can be represented as follows:
Na2+(g)→Na 3+(g)+ e −
- The third ionization potential is higher than both the first and second ionization potentials.
- This is because, as more electrons are removed from an atom, the remaining electrons experience an increasingly stronger effective nuclear charge, making it more difficult to remove additional electrons.
Generally, for alkali metals like sodium, the ionization potentials increase with each successive removal of electrons due to the increasing positive charge on the ion and the corresponding increase in the effective nuclear charge experienced by the remaining electrons.
The first ionization potential of noble gases and Alkali metals
Noble gases and alkali metals have distinct trends in their first ionization potentials due to their positions on the periodic table.

Noble Gases:
- Noble gases have relatively high first ionization potentials because it takes a significant amount of energy to remove an electron from a stable, fully-filled outer shell.
- They have a full outer electron shell this makes them highly stable and unreactive.
10Ne:[2He]:,2s2, 2p6
18Ar:[10Ne]:3s2,3p6
Alkali Metals:
- The First ionization energy of Alkali metals is lower than of all elements due to the easily loss of the valence electron.
- They have one electron in outermost shell
11Na :[10Ne]:,3s1 19K:[18Ar],4s1
Why the first ionization potential of noble gases is very high?
- Because they have complete electron shells, where their outer electron shells are fully occupied with electrons so they are in highly stable state that causes the difficulty in removing an electron from an atom that has strong electrostatic force of attraction between the positively charged nucleus and the electrons.
Why the first ionization potential of alkali metals is lower than of all elements?
- The lower first ionization potential of alkali metals is primarily due to two factors:
Large Atomic Size: Alkali metals have relatively large atomic sizes because they are located in the leftmost column of the periodic table. As you move down a group, the atomic size increases.
The valence electron in alkali metals is farther away from the nucleus.
- Single Valence Electron: The farther the electron is from the nucleus, the weaker the attractive force, making it easier to remove.
In summary, the combination of a large atomic size and a single valence electron makes alkali metals more prone (tend) to losing that electron and having a lower first ionization potential compared to other elements.
The ionization potentials of Magnesium
The electron configuration of magnesium is : Mg:[10Ne]3s2
Here are the approximate values for the ionization potentials of magnesium:
First Ionization Potential: The energy required to remove the outermost (highest energy level) electron.
Mg(g)→Mg + (g)+e − First Ionization Potentia ∆H1=+737 kJ/mol
12Mg:1s2,2s2,2p6, 3s2 12Mg+:1s2,2s2,2p6, 3s1
Second Ionization Potential: The energy required to remove a second electron after the first one has already been removed.
Mg + (g)→Mg 2+ (g)+e − Second Ionization Potential ∆H1=+1450 kJ/mol
12Mg+:1s2,2s2,2p6, 3s1 12Mg++:1s2,2s2,2p6
Third Ionization Potential: The energy required to remove a third electron after the first two have already been removed.
Mg 2+(g) → Mg3+(g)+ e− ∆H1=+7730 kJ/mol
12Mg++:1s2,2s2,2p6 12Mg+++:1s2,2s2,2p5
Why the second ionization of Mg is higher than the first one?
Due to the increasing of the effective nuclear charge (Zeff)
The third ionization potential of magnesium is much higher than of its first and second ones because it results in the breaking up of a completely filled energy level
Why the first ionization potential of K is lower than that of Ca?
The first ionization potential of potassium (K) is lower than that of calcium (Ca) due to differences in their atomic structures and electron configurations.
Potassium (19K):1s2.2s2,2p6,3s2,3p6,4s1
K has one electron in its outermost energy level (valence electron).
The removal of this valence electron involves breaking it free from a relatively weak attraction compared to electrons in inner energy levels.
Calcium (20Ca): 1s2.2s2,2p6,3s2,3p6,4s2
Ca has two electrons in its outermost energy level.
The lower first ionization potential of potassium compared to calcium is primarily due to the fact that potassium has a single valence electron, and the removal of this electron requires less energy compared to the removal of an electron from the outermost shell of calcium, which has two electrons in that shell.
Why the second ionization potential of K is much higher than that of Ca?
Due to differences in their electron configurations and the removal of electrons from different energy levels.
Potassium (19K):1s2.2s2,2p6,3s2,3p6,4s1
The second ionization involves breaking up of a completely filled shell which requires more energy.
Calcium (Ca): Calcium (20Ca): 1s2.2s2,2p6,3s2,3p6,4s2
While Ca has low second ionization potential because, in the case of potassium, the second ionization involves removing an electron from 4s1
The graduation of ionization potential in the periodic table
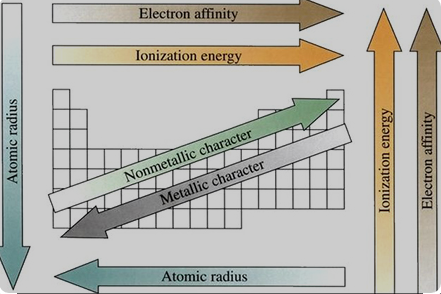
Across a Period (from left to right):
Ionization potential generally increases.
- This is because, within a period, the number of protons in the nucleus increases, leading to a higher effective nuclear charge.
- The increased positive charge exerts a stronger pull on the electrons, making them more difficult to remove.
- Electron shielding remains relatively constant within a period, so the increased positive charge dominates.
Down a Group (from top to bottom):
Ionization potential generally decreases.
- This is because, within a group, the outermost electrons are in higher energy levels (shells) that are farther from the nucleus.
- The electrons in higher energy levels experience less effective nuclear charge because they are shielded by inner electron shells.
- As you move down a group, additional electron shells are added, increasing shielding and reducing the attraction of outer electrons to the nucleus.
Exceptions:
There can be occasional anomalies, especially when comparing elements in adjacent groups.
For example, elements in Group 15 (nitrogen, phosphorus, etc.) may not follow the expected trend due to the stability associated with half-filled and fully-filled orbitals.
The relation between the ionization potential and the atomic radius
The ionization potential and atomic radius have an inverse relationship in the periodic table.
As you move across a period from left to right or down a group from top to bottom, the ionization potential generally increases, while the atomic radius tends to decrease.
Across a Period (from left to right):
Ionization potential generally increases.
Atomic radius generally decreases.
Down a Group (from top to bottom):
Ionization potential generally decreases.
Atomic radius generally increases.
In summary, there is an inverse relationship between ionization potential and atomic radius: as the atomic radius increases, the ionization potential tends to decrease, and vice versa.
The ionization potential of P is higher than of S although P precedes sulphur in the P.T
Let’s compare sulfur (S) and phosphorus (P) in terms of electronic configuration and ionization potential:
Electronic Configuration:16S :1s2 2s2 2p6 3s2 3p4
Phosphorus (15P): 1s2 2s2 2p6 3s2 3p3
Because the atom becomes more stable when the 3p sublevel is half-filled with electrons as in phosphorous atom and removing an electron from it it will decrease its stability
The ionization potential of 13Al is lower than of Mg although Al comes next Mg in the same period
Because the atom becomes more stable when the 3s sublevel is completely filled with electrons as in magnesium atom and removing an electron from it will decrease its stability
Let’s compare aluminum (Al) and magnesium (Mg) in terms of electronic configuration and ionization potential:
Electronic Configuration:
Aluminum (13Al):1s2 2s2 2p6 3s2 3p1
Magnesium (12Mg): 1s2 2s2 2p6 3s2
ionization potential of sulphur and Aluminum Let’s compare sulfur (S) and aluminum (Al) specifically in terms of their first ionization potentials:
Sulfur (S):Atomic Number: 16
Electronic Configuration:1s2 2s2 2p6 3s2 3p4
Aluminum (Al):Atomic Number: 13
Electronic Configuration: 1s2 2s2 2p6 3s 2 3p 1
First Ionization Potential of Sulfur is higher than that of Al
Due to:
- The ionization potential increases in period from left to write by increasing atomic number
- Aluminum is a metal, and metals tend to have lower ionization potentials than nonmetals.
Ionization potential of Lithium and Cs
First Ionization Potential of Lithium is higher than that of Cs.Why?
The ionization potential generally decreases down a group in the periodic table, and cesium is located below lithium in Group 1.
Let’s compare lithium (Li) and cesium (Cs) specifically in terms of their first ionization potentials:
Lithium (3Li):Electronic Configuration:
1s2 2s1
Cesium (55Cs)::
1s22s22p63s23p64s23d104p65s24d105p66s1
Why does cesium have a lower first ionization potential than lithium?
- The ionization potential generally decreases down a group in the periodic table, and cesium is located below lithium in Group 1.
- Atomic Size: Cesium is significantly larger than lithium so The outermost electron in cesium is farther from the nucleus compared to the outermost electron in lithium, reducing the electrostatic attraction between the outer electron and the nucleus.
Electron affinity
- The amount of energy released when an extra electron is added to a neutral gaseous atom
X(g) + e − → X − (g)
- Electron affinity is the energy change that occurs when an atom gains an electron to form a negatively charged ion (anion).
- The electron affinity is expressed in units of energy per mole, typically kilojoules per mole (kJ/mol) or electron volts (eV).
- The magnitude of electron affinity is high when the added electron makes the sublevel half-filled or completely filled where in both cases it helps in the stability of the atom
The graduation of electron affinity in The periodic table

Here are the general trends:
Across a Period (from left to right):Electron affinity tends to increase.
Due to :the increase of the atomic number leading to the decrease of atomic radius which makes it easier for nucleus to at attract a new electron.
As you move across a period from left to right, the atomic size generally decreases, and the effective nuclear charge increases.
Down a Group (from top to bottom):
Electron affinity tends to decrease.
Because: The increase in atomic number leading to the increase in The atomic size due to the addition of new electron shells and The ability of nucleus to accept an additional electron decreases
Why the electron affinity values for beryllium ,nitrogen and neon are close to zero ?
The electron affinity values for beryllium, nitrogen, and neon are close to zero that is for specific reasons related to their electronic configurations and atomic structures.
Beryllium (Be):1s2,2s2
Nitrogen(7N):1s2,2s2,2p3
Neon (10Ne):1s2,2s2,2p6
So the atom will be more stable when the sublevel :
2s is completely filled as in case of beryllium
2p is half-filled as in case of nitrogen atom
2p is completely-filled as in case of Ne atom
And the addition of an electron to any of them will decrease its stability
Why the electron affinity of chlorine (-348.6 KJ/mol ) is greater than that of fluorine (-348 KJ/mol) although chlorine comes next fluorine in the same group ?
Because fluorine atom is a smaller in size as it has smaller radius than chlorine atom so adding an electron suffer a strong repulsive force with the nine electron which decreases the released energy due to consuming a part of it to overcome the repulsive force
Electronegativity
- Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons when it is part of a compound.
- It is the ability of an atom to pull electrons towards itself in a chemical bond. Electronegativity values are assigned to each element on the Pauling scale or other scales.
The difference between electronegativity and electron affinity
- Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond.
- Electronegativity of elements is represented by relative values and it refers to a compound atom
- The increase of the relative values of the electronegativity means the increase in the ability of the element atom to attract the electrons of the chemical bonds
- The difference in electronegativity between elements determine the nature of the bonds between atoms
- Electron affinity is a measure of the energy change when an atom gains an electron.
- Electron affinity refers to an atom in a single state
In summary, electronegativity is a concept related to chemical bonding, while electron affinity is more directly associated with the process of gaining an electron to form an ion.
The graduation of electronegativity in the periodic table
How electronegativity changes across the periodic table:
Across a Period (from left to right):
Electronegativity increases from left to right due to:
The increase of atomic number leading to the decrease of atomic radius so the ability of atom to attract the electrons of the bond increase
Down a Group (from top to bottom):
Electronegativity decreases from top to bottom due to:
The increase in atomic number increases the atomic size due to the addition of new electron shells so the ability of atom to attract the electrons of bond decreases.
Notes:
-The Atoms of nonmetals group 7A (Halogens) are the greatest in the electronegativity while the atoms of the alkali metals of group 1A are the lowest in the electronegativity.
-Fluorine (F):is considered to be the most electronegative element while the cesium (Cs) is considered to be the lowest electronegative element .
References
Educational Websites:
Khan Academy (https://www.khanacademy.org/)
Chemguide (http://www.chemguide.co.uk/)
Online Databases:
PubChem (https://pubchem.ncbi.nlm.nih.gov/)
ChemSpider (http://www.chemspider.com/)
