Oxidation numbers: How to calculate the oxidation numbers
Introduction
This lesson delves into the principles of redox reactions, guiding you through the process of balancing equations by calculating changes in oxidation numbers. Learn how to identify oxidizing and reducing agents, explore practical examples, and gain a comprehensive understanding of the underlying mechanisms in chemical transformations.
The oxidation number (or oxidation state)
It is a number that refers to the electric charge (positive or negative ) tha the atom or ion would have in the compound in ionic or covalent compounds.
Positive and Negative Values:
- Oxidation numbers can be positive, negative, or zero.
- Positive oxidation numbers indicate electron loss, while negative oxidation numbers indicate electron gain.
Sum of Oxidation Numbers:
- In a neutral compound, the sum of oxidation numbers for all atoms is zero.
- In ions or charged species, the sum equals the overall charge of the species.
- The sum of the oxidation numbers in a compound is equal to the overall charge of the compound.
- The sum of the oxidation numbers in a polyatomic ion is equal to the charge of the ion.
Use in Redox Reactions:
- Oxidation numbers are crucial for balancing redox reactions. In a redox reaction, one element undergoes oxidation (loses electrons), and another undergoes reduction (gains electrons).
- Oxidation State Changes: In a redox reaction, the change in oxidation state reflects the transfer of electrons. The reducing agent donates electrons (undergoes oxidation), and the oxidizing agent accepts electrons (undergoes reduction).
The significance of oxidation numbers in ionic compounds
If the oxidation number is positive it indicates that:
A)In ionic compounds:
- The positive charges indicate that that atom lost electrons to form positive ion (cation)
b)In covalent compounds:
- The positive charges indicates the electronic shift in the chemical bond between the atoms is away from the less electronegative atom.
If the oxidation number is negative charge it indicates that:
A)In ionic compounds:
- The number of electrons that the atom gained to to give negative ion (anion).
b)In covalent compounds:
- The negative charge indicate that the electron shift in the chemical bond between the atoms is towards the most electronegative atom.
Rules to calculate the oxidation numbers
Assigning oxidation numbers involves following a set of rules to determine the hypothetical charge that an atom would have in a compound or ion.
Here are the general rules for calculating oxidation numbers:
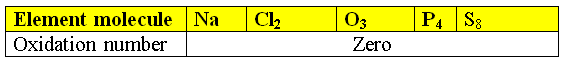
1)Elemental State:
- The oxidation number of an element in its elemental state is always zero.
- Also the molecule of multiple atoms Because the electronic shift in the bonds between the atoms equal
For example, the oxidation number of O2 or Na is 0.
2)Oxidation number of Monatomic Ions:
- The oxidation number of a monatomic ion is equal to its charge.
For example, the oxidation number of Na+ is +1, and the oxidation number of O2- is -2.

3)Oxidation number of Hydrogen:
- In most compounds, hydrogen has an oxidation number of +1.
- In binary compounds with active metals which are known as active metal hydrides its oxidation number is -1
However, in compounds with metals, hydrogen’s oxidation number can be -1, such as in LiH.

Note
- Hydrogen gas evolves at the anode (positive electrode) in electrolysis of molten sodium hydride while in electrolysis of acidified water it evolves at the cathode Why?
- Because the oxidation number of hydrogen in NaH molecule equals (-1) while in H2O molecule equals (+1)
Active metal hydrides:
- Ionic compounds formed form combination of an active metals with hydrogen where hydrogen has oxidation number(-1)
4)oxidation number of Oxygen:
- In most compounds, oxygen has an oxidation number of -2.
- In peroxides: oxidation number of -1.
- In Superoxide: oxidation number of -1/2
- In its compound with fluorine equals:+2 .

5)oxidation number of Alkali Metals (Group 1A):
- In compounds, alkali metals typically have an oxidation number of +1.
Alkaline Earth Metals (Group 2A):
- In compounds, alkaline earth metals typically have an oxidation number of +2.
Alkaline Earth Metals (Group 3A):
- In compounds, alkaline earth metals typically have an oxidation number of +3.

6) oxidation number Fluorine:
- Fluorine always has an oxidation number of -1 in compounds Give reason?
-Because its electronegativity is greater than that of all other elements

7)The Oxidation numbers of Halogens group 7A
The oxidation numbers of (halogens group 7A) in the most of their compound equals(-1)

8)Oxidation number Polyatomic Ions (atomic groups):
The sum of oxidation numbers in a polyatomic ion is equal to the charge of the ion.

9)Transition Metals:
The oxidation number of a transition metal in a compound can vary. It is often necessary to use information about the charges on other atoms in the compound or use other rules to determine the oxidation number of a transition metal.
- The algebraic sum of the oxidation numbers of the atomic group forming the molecule = zero
Example: in sodium chloride molecule NaCl:
The oxidation no. of sodium ion Na (+1) +The oxidation no. of chloride ion (-1)
So (+1) + (-1)= zero
- The algebraic sum of the oxidation numbers of the atoms that constitute the molecule of compound = zero
Example:
In the molecule :NH4NO2
NH4 atomic group carries +1 charge so its oxidation number =+1
NO2 atomic group carries (-1) charge so its oxidation number =-1
So the sum of oxidation numbers of the 2 atomic groups = (+1) + ( -1)=0
- The algebraic sum of the oxidation numbers of the different atoms in the poly atomic ion = the charges of ion (atomic group)
Example:
The hydroxide group OH-:
The oxidation number of oxygen=-2
The oxidation number of Hydrogen =+1
So the sum of oxidation numbers of Oxygen and hydrogen=(-2)+(+1)=-1
Examples
Oxidation Numbers in H₂O (Water):
- The oxidation number of hydrogen is usually +1.
- The oxidation number of oxygen is usually -2.
Therefore, in H₂O:
Each hydrogen has an oxidation number of +1.
Oxygen has an oxidation number of -2.
The overall sum is 0 (since it’s a neutral molecule).
Oxidation Numbers in NaCl (Sodium Chloride):
- The oxidation number of monatomic ions is equal to their charge.
Therefore, in NaCl:
Sodium (Na) has an oxidation number of +1.
Chlorine (Cl) has an oxidation number of -1.
The overall sum is 0 (since it’s a neutral compound).
Oxidation Numbers in HCl (Hydrochloric Acid):
- Hydrogen usually has an oxidation number of +1.
- Fluorine always has an oxidation number of -1.
Therefore, in HCl:
- Hydrogen has an oxidation number of +1.
- Chlorine has an oxidation number of -1.
The overall sum is 0 (since it’s a neutral compound).
Oxidation Number sulphur in SO₄²⁻ (Sulfate Ion):
- Oxygen usually has an oxidation number of -2.
- The sum of oxidation numbers in a polyatomic ion is equal to the charge of the ion.
Therefore, in SO₄²⁻:
- Each oxygen has an oxidation number of -2.
- The sum of oxidation numbers = 4(-2) + x = -2 (charge on sulfate ion).
- Solve for x: x = +6.
- The oxidation number of sulfur (S) in SO₄²⁻ is +6.
Steps to calculate the oxidation numbers of unknown element in compound or atomic group
Calculating the oxidation number of an unknown element in a compound or atomic group involves following a set of systematic steps.
Here are the general steps:
Identify Known Oxidation Numbers:
Review the oxidation number rules to identify the known oxidation numbers of elements in the compound or group.
Apply Rules to Known Elements:
Determine the oxidation numbers of the known elements in the compound or group using the established rules.
For example, hydrogen is typically +1, oxygen is typically -2, and alkali metals are typically +1.
Use Sum Rule:
Apply the sum rule, which states that :
“The sum of oxidation numbers in a compound is equal to the overall charge of the compound”.
- If the compound is neutral, the sum of oxidation numbers is zero.
Assign Unknown Element’s Oxidation Number:
- Assign the oxidation number of the unknown element (let’s call it X) based on the sum rule and the known oxidation numbers.
- If the compound is neutral, the sum of oxidation numbers is zero.
- If the compound has a charge, the sum of oxidation numbers equals the charge.
Be aware of special cases and exceptions. Some elements, especially transition metals, can have variable oxidation states.
Let’s illustrate these steps with an example:
Example:
Calculate the oxidation number of sulfur in H₂SO₄ (sulfuric acid):
Known oxidation numbers: Hydrogen is +1, and oxygen is -2.
Apply rules:
H₂ has hydrogen with oxidation number +1, and the sum rule implies sulfur must have an oxidation number of +6.
H2SO4=0
2×1+S+(-2)x4=0
2+S-8=0
S-6=0
S= +6
Example
Calculate the Oxidation Number of Nitrogen in NH₃ (Ammonia):
Known oxidation numbers: Hydrogen is +1.
Apply rules:
The sum of oxidation numbers is zero in a neutral molecule.
Let x be the oxidation number of nitrogen.
Equation: x + 3(+1) = 0
Solve for x: x = -3
The oxidation number of nitrogen in NH₃ is -3.
Example
Calculate the Oxidation Number of Chromium in Cr₂O₇²⁻ (Dichromate Ion):
Known oxidation numbers: Oxygen is usually -2.
Apply rules:
The sum of oxidation numbers is equal to the charge of the ion (-2 in this case).
Let x be the oxidation number of chromium.
Equation: 2x + 7(-2) = -2
Solve for x: x = +6
The oxidation number of chromium in Cr₂O₇²⁻ is +6.
Example
Calculate the Oxidation Number of Sulfur in H₂SO₃ (Sulfurous Acid):
Known oxidation numbers: Hydrogen is +1, and oxygen is usually -2.
Apply rules:
The sum of oxidation numbers is zero in a neutral molecule.
Let x be the oxidation number of sulfur.
Equation: 2(+1) + x + 3(-2) = 0
Solve for x: x = +4
The oxidation number of sulfur in H₂SO₃ is +4.
Example
Calculate the Oxidation Number of Phosphorus in PF₃ (Phosphorus Trifluoride):
Known oxidation numbers: Fluorine is always -1.
Apply rules:
The sum of oxidation numbers is zero in a neutral molecule.
Let x be the oxidation number of phosphorus.
Equation: x + 3(-1) = 0
Solve for x: x = +3
The oxidation number of phosphorus in PF₃ is +3.
Example
Calculate the Oxidation Number of Chlorine in Cl₂O₇ (Perchloric Acid):
Known oxidation numbers: Oxygen is usually -2.
Apply rules:
The sum of oxidation numbers is zero in a neutral molecule.
Let x be the oxidation number of chlorine.
Equation: 2x + 7(-2) = 0
Solve for x: x = +7
The oxidation number of chlorine in Cl₂O₇ is +7.
Example
Calculate the Oxidation Number of Carbon in CO₃²⁻ (Carbonate Ion):
Known oxidation numbers: Oxygen is usually -2.
Apply rules:
The sum of oxidation numbers is equal to the charge of the ion (-2 in this case).
Let x be the oxidation number of carbon.
Equation: x + 3(-2) = -2
Solve for x: x = +4
The oxidation number of carbon in CO₃²⁻ is +4.
Example
Calculate the Oxidation Number of Iodine in HIO₄ (Periodic Acid):
Known oxidation numbers: Hydrogen is +1, and oxygen is usually -2.
Apply rules:
The sum of oxidation numbers is zero in a neutral molecule.
Let x be the oxidation number of iodine.
Equation: (+1) + x + 4(-2) = 0
Solve for x: x = +7
The oxidation number of iodine in HIO₄ is +7.
Example
Calculate the Oxidation Number of Manganese in MnO₄⁻ (Permanganate Ion):
Known oxidation numbers: Oxygen is usually -2.
Apply rules:
The sum of oxidation numbers is equal to the charge of the ion (-1 in this case).
Let x be the oxidation number of manganese.
Equation: x + 4(-2) = -1
Solve for x: x = +7
The oxidation number of manganese in MnO₄⁻ is +7.
Example
Calculate the Oxidation Number of Sulfur in SO₂ (Sulfur Dioxide):
Known oxidation numbers: Oxygen is usually -2.
Apply rules:
The sum of oxidation numbers is zero in a neutral molecule.
Let x be the oxidation number of sulfur.
Equation: x + 2(-2) = 0
Solve for x: x = +4
The oxidation number of sulfur in SO₂ is +4.
Example
Calculate the Oxidation Number of Iron in Fe₂O₃ (Iron(III) Oxide):
Known oxidation numbers: Oxygen is usually -2.
Apply rules:
The sum of oxidation numbers is zero in a neutral molecule.
Let x be the oxidation number of iron.
Equation: 2x + 3(-2) = 0
Solve for x: x = +3
The oxidation number of iron in Fe₂O₃ is +3.
Example
Calculate the Oxidation Number of Nitrogen in NO₃⁻ (Nitrate Ion):
Known oxidation numbers: Oxygen is usually -2.
Apply rules:
The sum of oxidation numbers is equal to the charge of the ion (-1 in this case).
Let x be the oxidation number of nitrogen.
Equation: x + 3(-2) = -1
Solve for x: x = +5
The oxidation number of nitrogen in NO₃⁻ is +5.
Example
Calculate the Oxidation Number of Phosphorus in H₃PO₄ (Phosphoric Acid):
Known oxidation numbers: Hydrogen is +1, and oxygen is usually -2.
Apply rules: The sum of oxidation numbers is zero in a neutral molecule.
Let x be the oxidation number of phosphorus.
Equation: 3(+1) + x + 4(-2) = 0
Solve for x: x = +5
The oxidation number of phosphorus in H₃PO₄ is +5.
Oxidation and Reduction reaction (redox reaction):

Oxidation process
- It is the process of losing electrons in chemical reaction causing increases in positive charge
Example:
- The metal loses one or more electron so its oxidation number increases
Na loses e- Na+
- So metal is oxidized(oxidation procures)
- Sodium is called reducing agent
Reduction :
- It is the process of gaining electrons in chemical reaction causing increases in negative charge
Example:
Nonmetal tend to gain electrons and change into anion negative ion
Cl gains e- Cl–
- So its oxidation number decreases
- It is reduced (reduction process)
- Chlorine is called oxidizing agent

How to calculate the change in of oxidation number in the oxidation-reduction reaction
The advantages of using oxidation numbers is that they can help in determining the type of chemical changes in elements during the chemical reactions
To calculate the change in oxidation number in an oxidation-reduction (redox) reaction, you need to identify the atoms that undergo changes in oxidation state (oxidation number).
Here are the general steps:
Assign Initial Oxidation Numbers:
Identify and assign the oxidation numbers for each atom in both the reactants and the products before the reaction occurs.
Follow the rules for assigning oxidation numbers.
Identify Oxidized and Reduced Species:
Determine which atoms are oxidized (increase in oxidation number) and which are reduced (decrease in oxidation number).
The oxidized species loses electrons, while the reduced species gains electrons.
Calculate Change in Oxidation Number:
For each atom undergoing a change in oxidation number, calculate the difference between the initial and final oxidation numbers.
The change is typically represented as Δoxidation number.
Determine the Number of Electrons Transferred:
- The change in oxidation number is directly related to the number of electrons transferred during the reaction.
- For each atom undergoing a change, the absolute value of the change in oxidation number corresponds to the number of electrons gained or lost.
Write the Half-Reactions:
- Separate the redox reaction into two half-reactions: one for oxidation and one for reduction.
- In the half-reactions, include the electrons as reactants or products based on the change in oxidation number.
Balance the Number of Electrons:
- Ensure that the number of electrons lost in the oxidation half-reaction is equal to the number of electrons gained in the reduction half-reaction.
- Adjust coefficients as needed to balance the number of electrons.
Here’s an example to illustrate these steps:
Example:
Let’s consider the reaction between hydrogen gas (H₂) and oxygen gas (O₂) to form water (H₂O):
Which of the following represents oxidation and reduction process
2H2(g)+O2(g)→2H2O(l)
1)Assign Oxidation Numbers:
In H2, hydrogen has an oxidation number of 0.
In O2, oxygen has an oxidation number of 0.
2)Determine Changes in Oxidation Numbers:
Hydrogen goes from an oxidation number of 0 to +1 in H2O.
Oxygen goes from an oxidation number of 0 to −2 in H2O.
3)Identify Oxidized and Reduced Species:
Hydrogen is oxidized (loses electrons) as it goes from H2 to H2O.
Oxygen is reduced (gains electrons) as it goes from O2 to H2O.
4)Write Half-Reactions:
Oxidation Half-Reaction:
2H2(g)→4H+(aq)+4e−
Reduction Half-Reaction:
O2(g)+4e− →2O2−(aq)
5)Balance the Equations:
The number of electrons in the oxidation half-reaction must equal the number in the reduction half-reaction.
Here, both equations are already balanced.
Combine Half-Reactions:
2H2(g)+O2(g)→2H2O(l)
In this example, hydrogen undergoes oxidation, losing electrons, while oxygen undergoes reduction, gaining electrons.
Example:
Mention which of the following changes represents an oxidation or a reduction process and which change neither oxidation nor reduction?
- N2O4 ⇒ NO2
? -2 ⇒ ? -2
N2O4 ⇒ NO2
2N+4(-2)=0⇒N+2(-2)=0
2N-8=0 ⇒ N-4=0
2N=+8 ⇒ N=+4
N=+4 ⇒ N=+4
No change

Neither oxidation nor reduction because there is no change in the oxidation number of nitrogen
CO2 ⇒ CO
? -2 ? -2
CO2 ⇒ CO
C+2(-2) ⇒ C+1(-2)
C+(-4)=0 ⇒ C-2=0
C=+4 ⇒ C=+2
Reduction: oxidation no. decreases
Carbon is reduced so its oxidation number decreases
- ClO– ⇒ ClO32–
The solution:
? -2 ⇒ ? -2
ClO– ⇒ ClO32-
Cl+1(-2)=-1 ⇒ Cl+3(-2)=-2
Cl-2=-1 ⇒ Cl-6=-2
Cl=+1 ⇒Cl=+4
Chlorine oxidized because its oxidation number increases
Steps to calculate the change of the oxidation number in redox reaction
Calculating the change in oxidation number in a redox reaction involves identifying the atoms that undergo changes in oxidation state (oxidation number) and determining the magnitude of these changes.
Here are the general steps to calculate the change in oxidation number in a redox reaction:
Step 1: Identify the Redox Reaction
- Identify the redox reaction and make sure it is balanced.
- A redox reaction involves the transfer of electrons between reactants, resulting in changes in oxidation numbers.
Step 2: Assign Initial Oxidation Numbers
- For each atom in both the reactants and the products, assign the initial oxidation numbers.
- Use the rules for assigning oxidation numbers, such as the rules outlined earlier.
Step 3: Identify Oxidized and Reduced Species
- Determine which atoms are oxidized (increase in oxidation number) and which are reduced (decrease in oxidation number).
- The oxidized species loses electrons, while the reduced species gains electrons.
Step 4: Calculate Change in Oxidation Number
- For each atom undergoing a change in oxidation number, calculate the difference between the initial and final oxidation numbers.
- The change is typically represented as Δoxidation number.
Step 5: Determine the Number of Electrons Transferred
- The change in oxidation number is directly related to the number of electrons transferred during the reaction.
- The absolute value of the change in oxidation number corresponds to the number of electrons gained or lost.
Step 6: Write the Half-Reactions
Separate the redox reaction into two half-reactions:
- one for oxidation and one for reduction.
- In the half-reactions, include the electrons as reactants or products based on the change in oxidation number.
Step 7: Balance the Number of Electrons
- Ensure that the number of electrons lost in the oxidation half-reaction is equal to the number of electrons gained in the reduction half-reaction.
- Adjust coefficients as needed to balance the number of electrons.
Step 8: Combine Half-Reactions
Combine the balanced half-reactions to form the overall balanced redox reaction.
Balancing redox reactions by calculating the change in oxidation numbers:
Example 1:
1)Balancing the Redox Reaction
H2+O2→H2O
2)Assign Initial Oxidation Numbers:
H2: 0 (hydrogen in its elemental state)
O2: 0 (oxygen in its elemental state)
H2O: H: +1, O: -2
3)Identify Oxidized and Reduced Species:
H2 is oxidized (hydrogen’s oxidation number increases from 0 to +1).
O2 is reduced (oxygen’s oxidation number decreases from 0 to -2).
4)Calculate Change in Oxidation Number:
For H in H2:0 to +1 = +1 (change is +1)
For O in O2: 0 to – 2 = -2 (change is -2)
5)Determine the Number of Electrons Transferred:
For H: 2 electrons transferred (2-0)
For O: 1 electron transferred (0-(-2))
6)Write Half-Reactions:
Oxidation:
H2→2H+ +2e−
Reduction:
O2+4e−→2O2−
7)Balance the Number of Electrons:
Multiply the oxidation half-reaction by 2 to balance the number of electrons.
Combine Half-Reactions:
2H2+O2→2H2O
