The metallic, nonmetallic property, acidic and basic property
Introduction
Challenge your understanding of chemical properties with our “Give Reasons” quiz. Explore the reasons behind the behavior of metals, nonmetals, and other elements in various chemical processes
The metallic and nonmetallic property
At the beginning of the nineteenth century Berzelius, a Swedish chemist classified elements according to physical properties into two groups :Metals and nonmetals
Classification of elements:
According to atomic structure elements are classified into 3 groups:
1) Metals 2)nonmetals 3)metalloids
Metals Properties:
- Their valence shell has less than half its capacity of electrons
- The have large atomic radius which leads to small values for ionization energy and electron affinity
- They are electropositive elements due to their tendency to lose electrons of the valence shell and change into positive ions to reach the structure of the nearest noble gas
- Good conductors of heat and electricity due to the mobility of their few valence electrons which can transfer from one position to another in the metal.
Examples: Iron (Fe), copper (Cu), aluminum (Al), gold (Au).
Nonmetals Properties:
- Their valence shell has more than half its capacity of electrons
- They have small atomic radius which leads to high values for ionization energy and electron affinity
- The are electronegative elements due to their tendency to gain electrons to from negative ions that have the same electron structure of the nearest noble gas
- They are electric insulator where they don’t conduct electricity because their valence electrons are strongly bond to the nucleus so it is difficult for these valence electrons to be transferred.
Examples: Oxygen (O), nitrogen (N), fluorine (F), sulfur (S).
Metalloids (Semimetals) Properties:
- Have properties intermediate between metals and nonmetals have metallic appearance and the most properties of nonmetals .
- Their electronegativity in intermediate between metals and nonmetals
- Their electric conductivity is less than metals and more than nonmetals
- They are used in manufacturing some electronic devices parts such as transistors as the are semiconductors
Examples: Silicon (Si), germanium (Ge), arsenic (As), boron (B).
The graduation of metallic and nonmetallic property in the periodic table:
Across Periods (Left to Right):
- Metallic character tends to decrease across a period from left to right.
- As you move towards the right, elements become less metallic and more nonmetallic in character.
- Strongest metals in group 1A then the metallic property decreases gradually by increasing the atomic number till reach metalloids then the highest nonmetallic property in group 7A
Down Groups (Top to Bottom):
Metallic Properties:
- Metallic character tends to increase down a group.
- As you move down, the elements become more metallic due to their large atomic radius ad the low ionization potential and electron affinity.
- Nonmetallic character tends to decrease down a group.
Why Cs is considered the most active metal?
- Cesium (Cs) is considered one of the most reactive metals, and there are several reasons for its high reactivity:
- Cesium has low ionization energy and cesium’s outermost electron is relatively far from the nucleus due to its location in the last period (group 1, period 6) of the periodic table, it experiences weak attractive forces from the nucleus. As a result, cesium can easily lose its outermost electron, making it highly reactive.
- Large Atomic Size: Cesium has a large atomic radius This makes it easier for the outermost electron to be lost during chemical reactions.
Give reasons: F is considered the most active nonmetal?
- Fluorine (F) is considered the most active nonmetal for several reasons:
- Because the nonmetallic property decreases at the same group by increasing the atomic number and it placed at the top of the right side of the table (the most electronegative nonmetal)
The graduation of metallic and nonmetallic property in the third period
- As the atomic number increases the basic property decreases and the acidic property increases
Types of oxides
- Oxides are compounds composed of oxygen and another element (or elements).
- Depending on the nature of the other element(s) and their bonding with oxygen, oxides can be classified into three main types:
1)Acidic oxides,
2)basic oxides
3)amphoteric oxides.
Acidic Oxides:
- They are nonmetallic oxides
- They are called Acidic oxide due to:
a)Dissolve in water forming oxygenated acids
- Acidic oxides react with water to form an acid.
CO2(g) + H2O(l) >> H2CO3
carbon dioxide water carbonic acid
SO3(g) + H2O(l) >> H2SO4
Sulphur trioxide water sulphuric acid
- The react with Alkalis forming salt and water
CO2(g) + 2NaOH(aq) >> Na2CO3(aq) + H2O(L)
carbon dioxide sodium hydroxide sodium carbonate water
Basic Oxides:
- They are metallic oxide known as basic oxide
- Some of basic oxides are not soluble of water such as CaO
- Others are soluble in water forming alkali solution such as Na2O and K2O
K2O+H2O >> 2KOH
Na2O+H2O >> 2NaOH
- They react with acids forming salt and water
Na2O + 2HCl >> 2NaCl + H2O
MgO(s) + H2SO4(aq) >> MgSO4(aq) + H2O(l)
Amphoteric Oxides:
- Amphoteric oxides can act as either acidic or basic.
- They React with acid as base and react with base as acid forming salt and water
The reaction between zinc oxide (ZnO) and sulfuric acid (H2SO4) involves the amphoteric nature of zinc oxide.
ZnO+H2SO4→ZnSO4+H2O
The reaction between zinc oxide (ZnO) and sodium hydroxide (NaOH) involves the amphoteric nature of zinc oxide.
ZnO + 2NaOH → Na2ZnO2 + H2O
Zinc oxide sodium hydroxide sodium zincate water
Graduation of Acidic and Basic Properties :
In groups which starts by a metal :
- The basic property of the oxide increases as the atomic number increases as in group (1A)
In the groups which start by a nonmetal :
- The acidic property of the oxide increases as the atomic number increases as in group 7A
The graduation of acidic property of halogens
- The acidic property of hydrogen compounds of group 17 (halogen) increases as the atomic number increases GR?
-Because the increase in atomic number in the group leads to the increase in the atomic radius of halogens so its attraction force to hydrogen atom decreases making it easier to ionized
The acidic and basic of the hydroxyl compounds
The hydroxy compounds
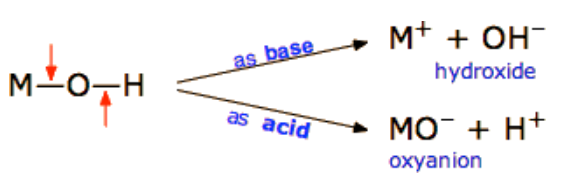
- The general formula of hydroxy compounds: MOH
The oxygenated acids and the bases
- Oxygenated acids and bases are classes of chemical compounds that contain oxygen and play significant roles in various chemical processes.
Let’s explore both oxygenated acids and bases:
The hydroxy compounds can be ionized by either ways:
A)As an acid:

The Hydroxy compounds can be ionized as acid If :
- The (M-O) bond is stronger than the (O-H) bond
- So the attraction force between (M+ an O–) is stronger than that between (H+ and O–)
MOH ⇔ MO– + H+
Oxygenated acid positive hydrogen ion
B) As base :

The Hydroxy compounds can be ionized as a base if:
- The (O-H) bond is stronger than the (M-O) bond
( The attraction force between (H+ an O—) is bigger than that between (M+ and O—)
MOH ⇔ M+ + OH–
Base
What happens if the strength of M-O bond is equal to O-H bond
- The compound may exhibit amphoteric behavior. Amphoteric substances can act as both acids and bases.
- The substance will be ionized as an acid or a base depending to the reaction medium
- React as a base in acidic medium and react as an acid in the basic medium
The basic property of sodium hydroxide compound:
- It is ionized as a base where sodium atom has a large volume and its ion has only one positive charge
- So the attraction between Na+ and O— decreases and the (O-H) bond is stronger than of the (Na-O) bond so OH- ion is produced
NaOH ⇔ Na+ + OH–

Why are the hydroxy compounds of the nonmetallic elements as chlorine ionized as an acid?
-Because the nonmetallic elements are characterized by the small atomic volume and the high charge which increase its attraction to O—ion and the (Cl-O) bond become stronger than the (O-H) bond so the positive hydrogen ion H+ is produced
The strength of oxygenated acids
- The oxygenated acids are represented by the following general formula
MOn(OH)m
- The strength of oxygenated acids increases as the number of nonbonded oxygen atom(On) with hydrogen increases
Number of Oxygen Atoms:
- In general, the presence of more oxygen atoms in an acid molecule tends to increase its acidity.
- The general formula “MOn(OH)m“ represents a class of compounds where “M” is a metal, “O” is oxygen, and “OH” is the hydroxyl group.
- The subscripts “n” and “m” represent the respective numbers of oxygen and hydroxyl groups in the compound.
This general formula is used to describe metal oxides or hydroxides.
Let’s break down the components:
“M” represents a metal element.
“O” represents oxygen.
“OH” represents the hydroxyl group.
“n” is the number of oxygen atoms in the compound.
“m” is the number of hydroxyl groups in the compound.
Examples
Silicate group: SiO4-

Ratio n:m : Zero
Number of nonbonded oxygen with hydrogen: Zero
Strength of the acid: weak
Phosphate group:PO43-

Ratio n:m : 1:3
Number of nonbonded oxygen with hydrogen:1
Strength of the acid: moderate
Sulphate group: SO42-
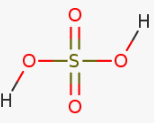
Ratio n:m : 2:2
Number of nonbonded oxygen with hydrogen: 2
Strength of the acid: strong
Perchlorate group: ClO4–

Ratio n:m : 3:1
Number of nonbonded oxygen with hydrogen: 3
Strength of the acid: The strongest
Quiz: Metallic and Nonmetallic Properties
1-According to Berzelius, how did he classify elements at the beginning of the nineteenth century based on physical properties?
2-List the three main groups elements are classified into based on atomic structure.
3-Provide two characteristics of metals related to their valence shell.
4-List two characteristics of nonmetals related to their valence shell.
5-Give one property that is intermediate between metals and nonmetals for metalloids.
6-What trend is observed in the metallic character across periods from left to right?
7-Explain the trend in metallic properties as you move down a group from top to bottom.
8-Why is Cesium (Cs) considered one of the most reactive metals? Provide at least two reasons.
9-Why is Fluorine (F) considered the most active nonmetal?
10 – As the atomic number increases in the third period, how does the metallic property change?
11 – Name the three main types of oxides and briefly describe each.
12- – Why are acidic oxides called “acidic,” and give an example reaction with water.
13- Provide one example of a basic oxide and its reaction with water.
14- Explain what amphoteric oxides can do and provide an example reaction.
15- – How does the acidic property of oxides change in groups starting with a metal and starting with a nonmetal?
16- Why does the acidic property of hydrogen compounds of halogens increase as the atomic number increases in Group 17?
17- What is the general formula for hydroxy compounds?
18-0How can hydroxy compounds be ionized as acids or bases?
19- What happens if the strength of the M-O bond is equal to the O-H bond in a compound?
20- According to the general formula “MOn(OH)m,” how does the strength of oxygenated acids change as the number of nonbonded oxygen atoms with hydrogen increases? Provide an example ratio and strength.
The answers
Here are the answers to the quiz:
- Classification of Elements:
According to Berzelius, he classified elements into Metals and Nonmetals based on physical properties.
- Classification According to Atomic Structure:
Metals, Nonmetals, Metalloids (Semimetals).
- Metal Properties:
Their valence shell has less than half its capacity of electrons.
They have a large atomic radius, leading to small values for ionization energy and electron affinity.
- Nonmetal Properties:
Their valence shell has more than half its capacity of electrons.
They have a small atomic radius, leading to high values for ionization energy and electron affinity.
- Metalloids (Semimetals):
Have properties intermediate between metals and nonmetals, including metallic appearance and most properties of nonmetals.
- Graduation Across Periods:
Metallic character tends to decrease across a period from left to right.
- Graduation Down Groups:
Metallic character tends to increase down a group.
- Cesium’s Reactivity:
Low ionization energy and large atomic size contribute to its high reactivity.
- Fluorine’s Reactivity:
Fluorine is considered the most active nonmetal because its nonmetallic property increases with atomic number, and it is the most electronegative nonmetal.
- Graduation of Metallic Property in the Third Period: – As the atomic number increases in the third period, the metallic property decreases, and the nonmetallic property increases.
- Types of Oxides: – Acidic oxides, basic oxides, and amphoteric oxides.
- Acidic Oxides: – They are called acidic oxides because they dissolve in water forming oxygenated acids.
- Basic Oxides: – Basic oxides are metallic oxides that can be soluble or insoluble in water, reacting to form alkali solutions or salts and water.
- Amphoteric Oxides: – Amphoteric oxides can act as either acidic or basic, reacting with acids as bases and bases as acids.
- Graduation of Acidic Property in Groups: – In groups starting with a metal, the basic property of oxides increases with atomic number. In groups starting with a nonmetal, the acidic property increases.
- Graduation of Acidic Property of Halogens: – The increase in atomic number in Group 17 leads to a larger atomic radius, reducing the attraction force to hydrogen, making ionization easier.
- Hydroxy Compounds: – The general formula is MOH.
- Oxygenated Acids and Bases: – They can be ionized as acids if the (M-O) bond is stronger than the (O-H) bond or as bases if the (O-H) bond is stronger.
- Amphoteric Behavior: – The compound may exhibit amphoteric behavior, acting as both an acid and a base depending on the reaction medium.
- Strength of Oxygenated Acids: – The strength increases as the number of nonbonded oxygen atoms (On) with hydrogen increases. Example ratios: – Ratio 0: Zero nonbonded oxygen, weak acid. – Ratio 2:2, strong acid. – Ratio 3:1, the strongest acid.
