Chemical equilibrium Reversible and irreversible chemical reactions
In this lesson we will study equilibrium in in the physical systems , equilibrium in chemical systems ,types of chemical reaction reversible or irreversible ,rate of chemical reactions and factors affecting of chemical reactions such as nature of chemical reactants , concentration of reactants , temperature , catalysts ,light and finally pressure
The equilibrium system:
It is apparently s stationary system but in reality dynamic .
[1]Equilibrium in the physical; systems :
-If we heated a water in a closed vessel :
1-there are two opposite processes (evaporation and condensation)
2-At the beginning of heating rate of evaporation is predominates which is followed by increase in the vapor pressure
Vapor pressure :the pressure due to water vapor in air at a certain temperature
3-the evaporation process continues until the vapor pressure equals saturated water vapor pressure
Saturated water vapor pressure:
The maximum water vapor pressure in air at a certain temperature
State of equilibrium :
The rate of evaporation =the rate of condensation
The number of water molecules which evaporate= the number of water vapor molecules condensate

Types of chemical reactions: according to equilibrium:
1)complete (irreversible) reaction 2) Reversible reaction
1)complete reaction:
– it goes in one direction (approximately forward) GR?
-because the products includes gas or precipitate that cant react to produce reactants
Examples:
1)adding sodium chloride solution to silver nitrate solution where a white precipitate of silver chloride is formed

2)adding a strip of magnesium metal to Hydrochloric acid solution hydrogen gas evolved

2)reversible reactions:
When adding one mole of acetic acid with one mole of ethyl alcohol , one mole of ester (ethyl acetate ) and one mole of water should be formed

If we put blue litmus paper it turns into red GR?
- Due to acetic acid where the reaction is reversible reaction which moves in both direction :forward and backward
-

forward reaction of acetic acid and ethyl alcohol

Chemical equilibrium in reversible reactions:
It is a dynamic system takes place when:
-the rate of forward reaction = the rate of backward reaction
-The concentration of the reactants and products are not changed
-The equilibrium remains unchanged since all reactants and products are still found in the medium of reaction (no gas evolve , no precipitate is formed and temperature and pressure not changed
Rate of chemical reaction:
-The change in concentration of reactants per unit time
-The concentration unit is expressed as : mole/liter
-The time : second or minute
– During the complete chemical reaction:
The concentration of the reactants decreases till consumed and the concentration of products increases
– During the reversible chemical reaction:
-The decrease in concentration of reactants and increasers the concentration of product occurs till the equilibrium sate


Types of chemical reaction according their speeds:
1)instantaneous reaction: take very short time
Reaction of silver nitrate with sodium chloride to form sparingly soluble precipitate of silver chloride
2)comparatively slow
Reaction between plants oil and caustic soda to form soap and glycerol
3)very slow: iron rusting : needs several months
Factors affecting the rate (speed)of chemical reactions:
1)Nature of reactants
2)concentration of reactants
3)reaction temperature
4)pressure
5)catalyst
1)Nature of reactants
a)Type of bonds in reactants b)surface area of reactants
Iconic bond covalent bond
A)Type of bonding in reactants:
– Iconic bond: reactants takes place very fast(instantaneous) GR? Because it occurs between IONS
Example: reaction between sodium chloride and silver nitrate
-Covalent bond: the reaction is slow GR? Because it occurs between molecules
Example: organic reactions
B)surface area exposed to reaction:
-Mass of block Zinc + volume of dilute hydrochloric acid : reaction is slow
-The same mass of zinc powder+ the same volume of hydrochloric acid:reaction is fast GR:
-because the exposed area to the reaction is case of block zinc is small then that in case of zinc powder
2)concentration of reactants
Increases of concentration of reactants cause increase of number of molecules of reactants that increases the collision between reactants molecules that increases the speed of the reaction
Waage and Guldberg(Norwegian scientists):
-they established the law of mass action that explain the the relationship between the concentration of reactants and the speed of chemical reaction
Law of mass action:
At constant temperature the rate of chemical reaction is directly proportional to the result of multiplication of the reactant concentration each raise to the power of the number of molecules or ions in the balanced equation
Excrement:
By gradual addition of the following reactants:
FeCl2(aq) + 3NH4SCN(aq) → Fe(SCN)3 (aq) +3NH4Cl(aq)
Iron III chloride ammonium thiocyanate iron III thiocynate
(pale yellow) (colorless) (blood red)
The color of the reaction mixture become blood red GR?
-due to the formation of iron III thiocynate
– If an excess of iron(III) chloride is added the red color of the solution increases indication the formation of more iron III thiocynate
-The equilibrium state occurs:
when: the rate of backward reaction (r2) = the rate of forward reaction (r1)

Note:
- [ ] :the concentration in unit( moles/liter)
- K1 and K2 :rate constants for the forward reaction and backward reaction
- At equilibrium: r1=r2

The product K1/K2 is a constant called Equilibrium constant of the reaction (Kc)
At equilibrium, the rates of the forward and reverse chemical reactions are equal:
aA + bB ⇌ cC + dD
The ratio between the concentrations of the products and reactants is a constant, known as the equilibrium constant, Kc:
Kc = [C]c[D]d/[A]a[B]b
In this equation, the square brackets indication concentration of the chemical species.
The exponents are the coefficients from the chemical equation.
The equilibrium constant for the reverse reaction, K’c, is given by the following:
K’c = 1/Kc = [A]a[B]b/[C]c[D]d
Example of law of mass action:
According to this law, “the rate of reaction of a substance is proportional to the product of molar concentration of reactants at a constant temperature at any given time”. Molar concentration is called active mass.
Active mass is the number of moles dissolved in one litre of solution.
eg. CH3COOH + C2H5OH ➝ CH3COOC2 H5 + H2O
According to law of mass action, Rate of forward reaction
a [CH3COOH] [C2H5OH] = K1 [CH3COOH] [C2H5OH] When K2 = rate constant for backward reaction At equilibrium,
Rate of backward reaction a [CH3COOC2H5] [H2O] = K2 [CH3COOC2H5] [H2O]
Where K2 = rate constant for backward reaction
At equilibrium, Rate of forward reaction = Rate of backward reaction K2 [CH3COOH] [C2H6OH] – K2 [CH3COOC2H5] [H2O]

When to Use the Law of Mass Action
Remember, the law of mass action only applies in cases of dynamic equilibrium. Regardless of the arrows in a chemical equation, make sure the following statements are true:
- The chemical equation represents the reaction of a closed system. That is, there is no heat or mass entering or leaving the system.
- Temperature remains a constant. At equilibrium, temperature does not change. Similarly, the equilibrium constant for a reaction depends on temperature. It’s value at one temperature may differ from Kc at another temperature.
Law of Mass Action Examples
For example, write the equilibrium constant expression for the dissociation of sulfuric acid into hydrogen and sulfate ions:
H2SO4 ⇌ 2H+ + SO42-
Answer: Kc = [H+]2[SO42-]/[H2SO4]
For example, if you know Kc is 5×105 for the reaction:
HCOOH + CN− ⇌ HCN + HCOO−
Calculate the equilibrium constant for the reaction:
HCN + HCOO− ⇌ HCOOH + CN−
Answer: The second equation is the reverse of the first equation.
K’c = 1/Kc = 1/(5 x 105) = 2 x 10-6
Example: calculate the equilibrium constant of the reaction:
H2(g) + I2(g) → 2HI(g)
If the concentration of I2 ,H2 and HI are 0.221,0.221 and 1.563 mole/liter respectively
solution: The equilibrium constant for the reaction
is at a given temperature.
The equilibrium concentration of and are and respectively.
The equilibrium concentration of is:
solution:
Notes:
If 1)Kc<1:The reaction is backward reaction
Example: Solubility of silver chloride in water
AgNO3 Ag+ + NO3– Kc=1.7×10-10
The value of Kc indicates that the silver chloride sparingly soluble in water
2)if Kc>1 :the reaction is forward reaction
Example: H2(g) + Cl2(g) HCl (g) Kc =4.4 x 1032
3)The concentration of reactants pure water , solid substances and precipitate are not appear in equilibrium constant equation GR?
-Because their concentration is constants not affected by its quantities
4)at the same temperature the value of equilibrium constant doesn’t change
The concentration of reactants and products
3)Effect of temperature in the rate of chemical reaction:
Collision theory:
To have a chemical reaction molecules of reactants must collide with each other
-molecules oh high speed can react because they have high kinetic energy enough to breach the bonds in reactant molecules
– molecules must have a minimum kinetic energy to collide together
Activation energy: the minimum amount of energy that must be gained by the molecules to react at collision
Activated energy: the molecules which have kinetic energy equals or more than the activated energy
- So increases the temperature causes increase the activated molecules that cause increase the rate of reaction
- The rate of chemical reaction is doubled by increasing the temperature by 10 C
Excrement: the effect of temperature in the rate of chemical reaction:
-by placing of nitrogen dioxide gas flask (reddish brown) in cold water the reddish brown color decreases until become colorless
-when putting it at room temperature (25 c) the reddish brown color appears again
So the color degree increases as temperature increases as the following:
+heat
reddish brown colorless
So if an exothermic reaction has reached the equilibrium state decrease in temperature force the reaction to go in the forward direction to liberate heat
4)Effect of pressure in the rate of chemical reaction:
If the reactants and products are in gaseous state the concentration is expressed by using partial pressure
for the reaction : ΔH=-92Kj
4 moles reacts to form 2 moles from ammonia molecule
So the formation of ammonia gas is followed by a decrease in the number of moles and consequently a reduction in the volume
-by increasing the pressure or cooling on a gaseous reaction under equilibrium a shift in the direction of reducing the volume (or the direction in which volume is less) takes place
The equilibrium constant(partial pressure :Kp
So the formation of ammonia gas is followed by a decrease in the number of moles and consequently a reduction in the volume
-by increasing the pressure or cooling on a gaseous reaction under equilibrium a shift in the direction of reducing the volume (or the direction in which volume is less) takes place
The equilibrium constant(partial pressure :Kp

Example: write the Kp for the following equation
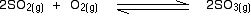

5)effect of catalyst:
Catalyst are substances that little amount of it cause a change in the rate of chemical reaction without itself being change or change equilibrium position
Why we use a catalyst ?to increase the rate of chemical to
Do decrease the consumed energy in industries and decrease the cost of products
-it decreases the activation energy of reaction so it used in 90% of industrial process (food ,petrochemicals industries)
– it is used in catalytic convertors in the car GR?
To convert the harmful gases of burning fuel to decrease air pollution
-the catalyst are: metals , metal oxides or compounds
Enzymes: proteins molecules produced in living cells act as catalyst in biological and industrial process
6)Effect of light:
In photosynthesis process chlorophyll in plants absorbs light and form carbohydrates in presence of carbon dioxide and water
-Photographic films contain silver bromide in a gelatinous layer
When light falls on it positive Ag ions accept electrons from negative bromide ions and converted to silver metal
-The increase the light intensity increases the amount of silver formed
Ag+ + e >>> Ag
Examples:
The so-called water-gas shift reaction:
, is useful in the production of hydrogen gas from steam and carbon monoxide. An equilibrium is reached at 700 K when and . W
hat is for the reaction at that temperature?
Step 1: Read through the given information and take note of the partial pressure of each reactant and product.
p and
Step 2: Write the expression for the reaction’s .
Kp=(pH2)(pCO2) / (pH2O)(pCO)
Calculating an Equilibrium Constant Kp Using Partial Pressures
Dark brown nitrogen dioxide gas fades to colorless dinitrogen tetroxide gas by the reaction:
When equilibrium is reached, pNO2=2.052 and pN2O4=0.474. What is Kp for the reaction?
Step 1: Read through the given information and take note of the partial pressure of each reactant and product.
and
Step 2: Write the expression for the reaction’s .
Step 3: Plug in the numerical values of the partial pressures, and calculate Kp.
Calculating an Equilibrium Constant Kp Using Partial Pressures
Partial pressures at equilibrium for the reaction :
are given in the table. What is for the reaction?
| Substance | Partial Pressure |
|---|---|
| H2 | 0.316 |
| I2 | 1.316 |
| HI | 4.368 |
Step 1: Read through the given information and take note of the partial pressure of each reactant and product.
These are conveniently found in the table.
Step 2: Write the expression for the reaction’s Kp.
Step 3: Plug in the numerical values of the partial pressures, and calculate Kp
References:
Read more on Sarthaks.com – https://www.sarthaks.com/707350/explain-law-of-mass-action-by-taking-the-following-example
