The galvanic cells , metal corrosions and metal protection
Galvanic cells
- A galvanic cell, is an electrochemical device that converts chemical energy into electrical energy. It consists of two half-cells, each containing an electrode and an electrolyte. The two half-cells are connected by a wire and a salt bridge.
- In a galvanic cell, a chemical reaction occurs at each electrode. At the anode (the negative electrode), oxidation occurs, which releases electrons into the wire. At the cathode (the positive electrode), reduction occurs, which consumes the electrons from the wire. The salt bridge maintains charge neutrality by allowing ions to flow between the two half-cells, which balances the charge buildup that would otherwise occur.
- The amount of electrical energy produced by a galvanic cell depends on the nature of the chemical reaction and the electrodes used. Galvanic cells are widely used in batteries, which can power electronic devices and provide backup power for various applications.
- Overall, galvanic cells are an important technology for the production of electricity, and they have a wide range of practical applications in many areas of science and engineering.
Kinds of galvanic cell:
Galvanic cells can be categorized as primary or secondary, depending on whether they are designed to be used only once (i.e., not rechargeable) or whether they can be recharged and reused.
Primary galvanic cells
They have a chemical energy and can converted into electrical energy through spontaneous irreversible oxidation-reduction reaction
-They can’t be recharged ,Once the chemical reactants are exhausted, the cell stops producing electricity and must be discarded.
Mention the disadvantages of the primary cells?
1)They are irreversible cell GR? because the can not recharged to return its chemicals to its initial state
2)they are stop when the anode consumed or the ions of cathode diminished
Mention the advantages of the primary cells?
1)They are found in dry from not in liquid GR? To be used easily in the the mobile equipment
2)They produce a constant potential for long time
3)They can be manufactured in small size such as: 1- Mercury cell 2-Fuel cell
Mercury cell:

1-Type:Primary galvanic cell
2-Shape:Small cylinder or disk
3-Electrolyte: Potassium hydroxide (KOH)
4-The anode(- electrode):Zinc (Zn)
5-The cathode (+ electrode):Mercury oxide ( HgO)
6-Reaction:
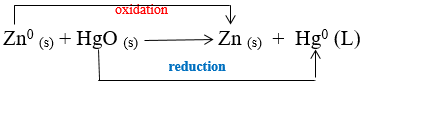
7-e.mf :E cell = emf cell = 1.35 V
8-Uses:
1)earphone
2)Clocks
3)Cameras GR? Because of its small size
9-Precautions after use:
-should be disposed after using by a safe way GR? -Because mercury is poisonous
Fuel cell

Hydrogen gas burns in air producing light and heat
2H2 + O2 (g) ⇒ 2H2O (v) + Energy
1-Type:Primary galvanic cell
2-Composition :
-Made of 2 poles each of them is similar to a hollow container lined by a layer of porous carbon GR ?
-because this layer connects between internal room and the electrolyte which exists inside ?
3-Used fuel:H2 gas and O2 gas supplied from external room
4-Electrolyte:Hot aqueous solution of potassium hydroxide ( KOH)
5-Anode reaction(oxidation):
2H2(g) + 4OH (aq) ⇒ 4H2O (v) + 4e– Eoxid=0.83V
6-Cathode reaction (reduction):
O2 (g) +2 H2O(v) + 4e– ⇒ 4OH– (aq) E red = 0.4 V
7-Total reaction:
2H2(g) +O2(g)⇒ 2H2O (v) E cell =1.23 V
8-emf:
E cell =emf cell = E oxid(Anode) + E red (Cathode)
= 0.83 + 0.4 = 1.23 V
9-Advantages of fuel cell:
The contents can not be consumed GR?
-because it is supplied with fuel from an external source
-it operates at high temperature so the produced water evaporates and can be condensed and reused as drinkable water
GR:
1))Fuel cell ( or mercury cell) is considered as a primary galvanic cell ?
- because the oxidation reaction in them is a spontaneous irreversible reaction
2)Fuel cell (or mercury cell ) is considered as an alkaline cell?
– because the electrolyte in them is potassium hydroxide(KOH)
3)Importance of fuel cells in space ships?
-because the fuel used in it is the same fuel used in launching rockets and reuse of water produced from its reaction after condensation as a drinkable water for astronauts
4)The difference in the performance of the fuel cell from the other galvanic cells?
– because its performance required continues supply the fuel and continuous removal of the products and the contents can not be consumed
Secondary cell
-It is a galvanic cell which is characterized by reversible oxidation reduction reaction and storing electric energy in the form of chemical energy and can be recharged
How to recharge the secondary cell ?
- by passing a direct electric current from external source in a direction opposite to its direction during the discharging process

Lead- acid battery
(the lead accumulator) is known as car battery GR?
- Because it is the most suitable kind for cars

–It consists of 6 or more cells connected in series
1-Type:Secondary galvanic cell
2-Composition :
-The lead-acid battery is made of a container of solid rubber or plastic(polystyrene) GR? Because it is no affected by sulphuric acid(electrolyte)
-plate of lead network which are separated from each other by insulating sheets and all of them are dipped in diluted sulphuric acid
3-Electrolyte:
Diluted sulphuric acid ( H2SO4)
H2SO4 (aq) ⇒ 2H+ (aq) + SO42- (aq)
4-The anode(-):Lead network filled with spongy Lead (Pb0)
5-The Cathode (+ electrode):Lead network filled with a paste of lead dioxide (PbO2)
6-Discharging reactions:
Lead accumulator discharging
A process of converting the chemical energy saved in the accumulator into electric energy through a spontaneous E-reduction
-on starting the car the lead accumulator acts as a galvanic cell in which :
-At the anode:
oxidation : Pb0 (s) + SO42-(aq) ⇒ PbSO4 (s) + 2 e_ E0 oxid=0.36
-At the cathode:
reduction : PbO2 (s) + 4H+ (aq) + 2e– ⇒ PbSO4 (s) + 2 H2O (L)
The total reaction:
Discharge: Pb0 (s) +PbO2 (s) +4H+(aq) +SO42-(aq) ⇒ 2PbSO4 (s) +2H2O (L)
E cell=2V approximately
7- Charging reaction:
-Using the car battery for a long time decreases the intensity of the electric current produced GR?
-because it leads to : increasing the dilution of sulphuric acid by water which is produced from the discharging reaction
– converting the anode material(Pb0) and the cathode material (pbO2) into lead (II) sulphate PbSO4 so the battery needs to be recharged that is means that the galvanic cell will be converted into electrolytic cell through inducing a nonspontaneous oxidation reduction reaction in a direction opposite to the direction of the spontaneous reaction which occur during discharging process
Lead accumulator charging : connecting the 2 electrodes of the accumulator with an external source of direct current its potential is slightly higher than the potential of the accumulator that leads to inducing a nonspontaneous oxidation-reduction reaction in opposite to the spontaneous reaction in discharging
–conversion of lead(II) sulphate PbSO4 into lead (Pb0) at the cathode of electrolytic cell (the anode of the galvanic cell) and lead dioxide (PbO2) at the anode of the electrolytic cell (cathode of the galvanic cell)
-restoring the concentration of suphuric acid
The reaction:
– At the Cathode:
Reduction: PbSO4 (s) + 2e – ⇒ Pb0 (s) + SO4 2- (aq)
-At the anode:
Oxidation: PbSO4 (s) +2H2O ⇒ PbO2 (s) +4H+ (aq) + SO4 2-(aq) +2e–
The total reaction:
2PbSO4 (aq) + 2H2O charge⇒ Pb0 (s) + PbO2 (s) + 4H+ (aq) +2SO4 2- (aq)
-The car dynamo is used in recharging the battery
Total reaction:
Pb0 (s) + PbO2 (s) +4H– (aq) +2SO42- (aq) ⇔ 2PbSO4 (s) +2H2O
8-e.m.f :
E cell = emf cell= E0 oxid (anode)+ E0 red (cathode)
=0.36 +1.69 =2 v approximatly
The battery contains 6 cells in series conction
Emf Battery = 2 x 6 =12 V
9-Detection of the battery state:
By using The hydrometer to measure the desity of sulphuric acid
-the battery is fully charged when: acid density:from(1.28: 1.30 g/cm3)
-requried to be recharged when:acid density less than 1.20 g/cm3
GR:
1)the secondary cell are considered to be energy storing batteries?
-because on charging the save the electric energy as chemical energy which can be converted into electric energy when needed
2)Lead accumulator is considered as an acidic battery ?
-because the electrolyte in it is diluted sulphuric acid
3) Car battery acts as a galvanic cell as well as an electrolytic cell ?
–it is a galvanic cell on discharging (chemical energy changes into electric energy)and electrolytic cell on recharging (electric energy changes into chemical energy)
4)The car battery represents as reversible cell ?
-because when it supplied with direct electric current from external source its oxidation reduction are reversed to reduction reaction and vice versa
5)Lead –Acid battery needs to be recharged from time to time
-because the long us of it decreases the concentration of sulphuric acid and the conversion of anode material and cathode material into lead(II) sulphate sit i needs recharged to restore the concentration of acid and change the lead sulphate into lead and lead dioxide
6)The total potential of the lead accumulator is 12 v although the lead-acid cell potential is 2 v only?
-because the lead accumulator contains 6 cells connected in series
The Lithium ion battery
-It is a dry rechargeable with a small size and lighter
-It is used in mobile ,lab top and the modern car GR?
Because it is small size and lighter and store a large amount of energy
Its structure contains Lithium GR?
-Because lithium is the lightest metal and has the lowest potential reduction (-3.04V)
1-Type:Secondary galvanic cell
2-Composition:
It consists of metallic covers composed of 3 thin layers rolled in a spiral shape:
1)-The anode:LiC6
2)-The Cathode: LiCoO2
3)-An isolator between anode and cathode
– the 3 layers are dipped in the electrolyte (LiPF6)
3-Electrolyte: Anhydrous solution of lithium hexa fluoro phosphate (LiPF6)
4-The anode(negative electrode):Lithium graphite (LiC6)
5-The cathode (positive electrode):Lithium cobalt oxide (LiCoO2)
6-The isolator: A thin layer of plastic separates between the Anode and the Cathode and allows ions to passes though it
7-Discharging:
-At the anode:
LiC6 (s) ⇒ C6 (s) + Li + (aq) + e–
lithium graphite graphite
-At the Cathode:
reduction :CoO2 + Li + (aq) + e– ⇒ LiCoO2 (s)
cobalt(IV) oxide Lithium cobalt oxide
-By addition the 2 equations:
discharge: LiC6 (s) + CoO2 (s) ⇒ C6(s) + LiCoO2 (s)
lithium graphite cobalt(IV) oxide graphite Lithium cobalt oxide
8-Total reaction :discharge > and recharge <
LiC6 (s) + CoO2 (s) ⇔ C6(s) + LiCoO2 (s)
lithium graphite cobalt(IV) oxide graphite Lithium cobalt oxide
9-Electromotive force:
E cell = emf Battery = 3v
10-Uses :
-used in mobile phones .laptops and electric car GR?
-because it is a small, lighter, and store large amount of energy
Metal Corrosion
Rusting
any process of chemical corrosion of metals by environmental effects (atmospheric air O2 and water vapor)
GR: Metal rusting is undesirable oxidation-reduction reaction?
Because it causes deterioration of metallic structure especially that made of iron leading to economic lose (about ¼ production of ion is lost in the world)
GR: the metals differ from each other in their vulnerability to corrosion ?
because rusting of metals depends on their activity where the metals lie at the top of electromotive series(more active) are easy to rust than the metals lie at the bottom

-If the layer is coherent,hard nonporous ,adhering the metal surface and insoluble in water it protect the metal from further corrosion.
-If the layer is fragile ,porous and soluble in water the corrosion increases what happens in the presence of the metal in aqueous medium?
GR: The existence of gold in nature individually?
- Because it lies at the bottom of the electromotive series
GR: Difficulity of rusting of Al although it lies at the top of ems?
-because it reacts with atmospheric air forming nonporous layer(Al2O3) prevent further reaction with air
Mechanism of metal corrosion
The pure metals are difficult to corrode but presence of impurities causes corrosion GR?
-Because the contact between the less active metal with more active metal causes corrosion of more active metals due to the formation galvanic cell:
Example :
Steel corrosion
When a piece of iron exposed to cracking or breaking it forms a local galvanic cell in which:
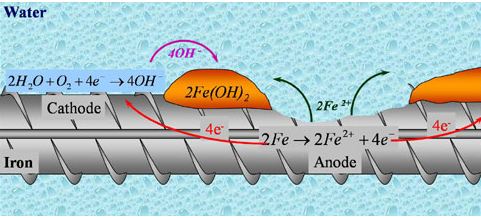
1)anode reaction: (iron piece) oxid
2Fe (s) 2Fe2+(aq) + 4e–
2)cathode reaction:(red.) carbon impurities , air
2H2O (L) +O2 (g) + 4e– ⇒ 4OH–(aq)
3)In the electrolyte:the water in which salts ion are dissolved
2Fe2+ (aq) + 4OH– (aq) ⇒ 2F2(OH)2 (s)
2Fe(OH)2(s) + ½ O2(g) + H2O ⇒ 2Fe(OH)3(s)
4)The total reaction:
2Fe(s) + 3H2O(L) + 3/2 O2(g) ⇒ 2Fe(OH)3(s)
The factors which cause metal corrosion
1) Heterogenity of alloys
It is difficult to prepare homogeneous alloys so a local galvanic cells are formed in alloy causes corrosion of more active metal
2)the connection of metal with another
Causes corrosion of more active metal GR?
due to the formation of galvanic cell
Examples : points of welding metals – using screws and rivets made from another metal
-Which rust first : Al with Cu and -Fe with Cu GR?
3)water ,oxygen and slats (external factors)
Methods of protection of Iron against rusting
1)painting it with an organic material(oil, varnich or primer (weak method)
2)covering it with corrosion resistant metals by 2 ways:
a)cathodic protection(cathodic cover) b)Anodic protection(Anodic cover)
a) Cathodic protection (cathodic cover):
covering the metal by a less active metal
Example: covering iron by Sn(tin) as in food cans
Disadvantages of the cathodic cover
On scratching iron is rusted faster than the uncovered Irion GR?
-Due to the formation of galvanic cell :the anode is iron and the cathode is the less active metal (tin)
b)Anodic protection: covering the metal by a more active metal
Example:
–steel covered by Zn or Mg in making ships
–plating iron by molten of Zn (Galvanization process)
Steel galvanization:
dipping the steel in molten of zinc to protect it from rusting
Advantages of the anodic cover:
On scratching the anodic cover (Zn) the protected (Fe) doesn’t start too be corroded until after the complete corrosion of the anodic cover that takes long time GR?
- Due the formation of galvanic cell :
- The cathode is the less active (Fe)
- The anode : is the more active(Zinc)
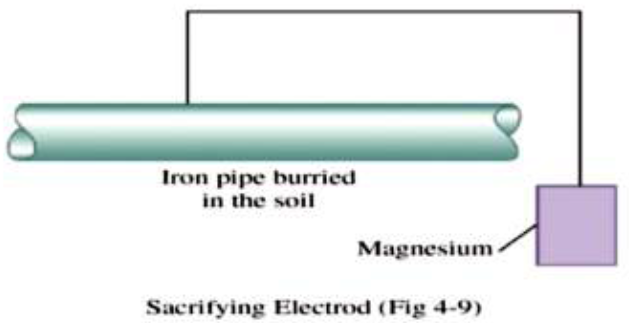
sacrifying electrode
It is the active metal which is corroded on connection with another less active metal where the more active metal acts as an anode to protect the less active (cathode) from corrosion
Example: iron pipes that are buried in the moist soil and the ship that in contact with salty water of the sea are exposed to corrosion HOW to protect them?
-by connecting them to more active metal than iron (like Mg ) which acts as anode and iron is cathode so Mg will be corroded instead of iron
GR:
1)The rate of rusting process of the food cans increases on scratching .
-due to the formation of local galvanic cell in which the anode is iron as the more active metal and the tin is cathode so iron rust faster
2)The anodic cover is more preferable than the cathodic cover
-because on scratching the anodic cove the protected metal doesn’t start to rust until the anodic cover is completely corroded that takes long time but on scratching the cathodic cover the protected metal rusts faster
3)Iron pipes buried in the moist soli are connected to zinc or (Mg) plates ?
-because Zinc or magnesium acts as a sacrifying electrode which is corroded instead of iron