The ionic equilibrium
The ionic equilibrium
The law of mass action and equilibrium
Chemical compounds
Ionic compounds:
Positive and negative ions are bonded by electrostatic forces
Example: Sodium chloride NaCl
-It dissociates completely in water into Na and Cl- ions
Covalent compounds
The atoms are bonded by covalent bonds
Example:
-Hydrogen chloride gas (HCl) ionizes completely in water
– Acetic acid
CH3COOH incompletely ionized in water
IONIZATION:
Process in which unionized molecules are changed into ions
Types of ionization
a) Complete ionization
b) incomplete ionization
1)Complete ionization:–
All unionized molecules are changed into ions(strong electrolyte)
HCl(aq) ⇔ H+(aq) + Cl–(aq)
2)incomplete ionization:
Small part of unionized molecules are changed into ions (weak electrolyte)
CH3COOH ⇔ H+ + CH3COO–
Question: compare between complete and incomplete ionization with example
Experiment: testing the electric conductivity of hydrogen chloride gas dissolved in benzene and pure acetic acid
Observation: the light bulb don’t light up in both cases
Conclusion:
The solution of HCl in benzene and pure acetic acid both don’t contains ions so they are nonelectrolytes
Expermint2:
The effect dilution of both hydrochloric acid and acetic acid
HCl acid (1M concentration) acetic acid(1 M concentration)
Then increases dilution to (0.01 M then 0.001M)
Observation:
In case of HCl : the illumination of the light bulb intense and not affected by dilution
In case of acetic acid:
Increases the light intense by increasing dilution
Conclusion:
HCl is strong acid ionizes completely in water not affected by dilution
HCl ⇔H+ + Cl–
-Acetic acid is weak acid
-it ionizes incompletely in water
-Its ionization increases by increasing dilution
CH3COOH ⇔CH3COO– + H+
GR: the ability of hydrochloric acid to conduct the electricity is not affected by dilution with water while acetic acid ability to conduct the electricity is affected by dilution
Hydronium ion (Hydroxonium ion)
(Hydrated proton)
Hydronium ion:
It is a positive ion produced from combination of water molecules with H+(proton)of ionized acid by coordinate bond
HCL(s) +H2O(l) ⇔H3O+(aq) +Cl–(aq)
Why hydronium ion is formed ?
-Due to the attraction of H+ ( of acid) with the lone pair electrons of oxygen atom in water molecules
GR: Hydronium ion is called hydrated proton?
IONIC equilibrium in weak electrolytes
There are two opposite process
– A weak (incomplete ) ionization takes place in weak electrolytes
Ionization of small amount and Recombination between ions to form molecules
CH3COOH(aq)+H2O(L) ⇔ CH3COO–(aq) + H3O+(aq)
A dynamic equilibrium state occurs:
CH3COO–(aq) + H3O+(aq) ⇔ CH3COOH(aq)+H2O(L)
Ionic equilibrium: the equilibrium state between molecules of a weak electrolytes and their ions
Question : compare between : chemical equilibrium and ionic equilibrium with example
GR: The law of mass action can not be applied to the strong electrolyte
Ostwald s law of dilution
He discovered (1888) the relation between :
The degree of ionization (dissociation(alpha ) and the concentration (C) mol/L in the weak electrolyte
Ostwald s law:
At a constant temperature the degree of ionization (ᾳ) of the weak electrolytes increases dilution
On dissolving of a weak monoprotic acid (HA) in water it ionizes incompletely.
HA(aq) ⇔+ (aq) + A– (aq)

By substitution in the law of mass action:
Equilibrium constant of weak acid(Ka)LIonization constant):
= the produced ions concentration / molecules concentration before ionization
Ka=[H+] [A–] / [HA]
Ka= Cᾳ . C ᾳ / C(1-ᾳ)
Ka= C2 ᾳ2 / C(1-ᾳ)
Ka=Cᾳ /(1-ᾳ)
The degree of ionization of weak acid (ᾳ) is negligible so
(1-ᾳ)=1

To calculate the concentration of weak base :

Example:
calculate the degree of ionization of 0.1 M hydrocyanic acid knowing that its equilibrium constant (ionization constant ) = 7.2 x 10-10
Answer:
ᾳ2=( Ka/Ca )2
ᾳ2=( 7.2 x10-10 /0.1 )2
then ᾳ = 8.5 x 10-5
Example: calculate the concentration of weak acid acetic acid CH3COOH if its ionization percentage = 0.42 % and its equilibrium constant Ka=1.8 x 10-5
Answer:
ᾳ= 0.42/100 =0.0042
Ca= Ka/ ᾳ2 =1.8 x 10-5 /(0.0042)2
Example:
a weak acid its degree of ionization = 2 x 10-2
in a 1L volume which contains 0.25 mol of it calculation the equilibrium constant of this acid.
Answer:
Concentration = No. of moles / volume (L)
Ca= 0.25 /1 = 0.25 M
Ka=Ca X ᾳ2 =
0.25 x (2×10-2)2
= 1 x 10-4
Example:
a weak acid its degree of ionization = 0.008 (at 25 c) in 0.15 M solution
Calculate its degree of ionization in 0.1 M solution at the same temperature what would you deduce
Answer:
Ka value is constant at constant temperature
C1 (ᾳ1)2 = C2 (ᾳ2)2
0.15 x (0.008)2 =0.1 (ᾳ2)2
(ᾳ2 = 0.15 x (0.008)2 /0.1
= 0.00098
deduction: the degree of ionization increases by increasing the dilution
To calculate of hydronium ion(H3O+) concentration in weak acid:
Acetic acid ionizes in water as:
CH3COOH + H2O ⇔ CH3COO– + H3O+
So Ka= [CH3COO] [H3O+] /[CH3COOH]
The no. of moles of H3O+= the No. of moles of CH3COO–
[H3O+] = [CH3COO–]
So Ka = [H3O+]2 / [CH3COOH]
ᾳ of weak acid is negligible value
so : acid concentration before ionization = acid concentration at equilibrium
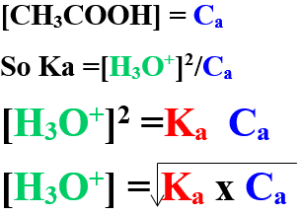
To calculate Hydronium ion concentration [H3O+] in a weak acids by knowing its degree of ionization (ᾳ):

Example:
calculate hydration ion concentration in a 0.1M solution of acetic if its ionization percentage =1.34 %
Answer: ᾳ =1.34/100
= 1.34 x 10-2
[H3O+] = ᾳ Ca = 1.34 x 10-2 x 0.1
= 1.34 x 10-3 M
To calculate of hydroxyl ion con.[OH–] in weak base solution:
Example:
NH3 gas dissolves in water giving a weakly ionized basic
NH3(g) + H2O(L) ⇔NH4+(aq) + OH– (aq)
Kb=[NH4+] [OH–] / [NH3]
No. of HO– ions = No. of NH4+ ions
[NH4+] = [OH–]
Kb = [OH–]2 / [NH3]
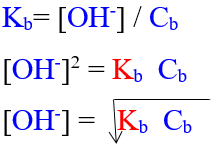
Example:
calculate hydroxyl ionization in 0.2 M solution of methyl amine CH3NH2 (at 25C) if equilibrium constant =3.6 x 10-4
