Uses of First Transition Elements (Economic importance)
Economic importance of first transition elements
The Presence of First transition elements in earth crust
1-They form about 7% of the weight of the earth’s crust but these elements have great economic importance. 2-(Fe) is highest value (6.3) and (Sc) is a smallest value (0.0026) so its rare transition element
The usage of The First Transition Series
Scandium (21Sc)
1-very small amounts on a wide range on the Earth’s crust 2-Sc+Al= alloy characterized by hardness and lightness so It is used in the MiG fighter aircraft industry GR? 3) It is added to mercury vapors lamps to give high-efficiency light like to sunlight so It is used in the photographing television during the night

Titanium 22Ti
Strong element of rigidity such as steel and less dense than steel
1-Titanium aluminum alloys are used in aircraft and spacecraft industry GR?
-because it maintains the solidity (durability) in high temperatures at a time when the hardness of aluminum decreases
2-In Dental implant and artificial joints GR?
-because the body not reject it and not cause poisoning
3-Titanium dioxide TiO2 :used in compounds of protection from the sun rays GR ?where its Nano particles prevent the effect of ultraviolet on the skin
| titanium aluminum alloy | titanium joints | titanium dental |
|
|
|
|
Vanadium 23V
-Vanadium with the steel gives a high hardness alloy and has high ability to resist corrosion, so it is used in the manufacturing of car springs industry GR? .
– Vanadium penta oxide (V2O5 ) is used as:
1-dyes in ceramic and glass industry
2-Catalyst in the strong magnetic conductors
|
|
 |
| used as dyes in ceramic and glass industry | vanadium steel alloy in car springs in car spring |
Chromium 24Cr
1) It has a high degree of chemical activity but it resists the atmospheric air GR?
due to the formation non-porous a layer of oxide with large size above its surface that prevent the continuation of chromium reaction with oxygen atmosphere.
Uses of Cr :
| Metal plating :chromium plating | tuning leather |
 |
 |
The most important compounds
1)-Chromium III oxide: ( Cr2O3): in making dyes
2)-Potassium dichromate ( K2Cr2O3) :it is used as an oxidizing agent.
 |
|
| Ni and Cd battery | Cr2O3 in deys |
Manganese 25Mn
1-It is used in the form of alloys or compounds not used in its pure state GR ? -because it is a brittle element 2-It is used with iron alloys in making the railway tracks GR?.
-because it is harder than steel. 3- It is used with aluminum alloys in packages of soft drinks vessels (cans) GR ? because its resistance to corrosion. The most important compounds:
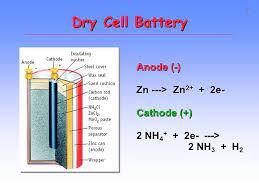
A-Manganese dioxide: ( MnO2) : used as strong oxidizing agent and making dry cell
B-Potassium permanganate: ( KMnO4) : used as an oxidizing substance and disinfectant.
c- Manganese II sulphate : ( MnSO4): it is used as fungicide
|
Mn and Al alloy used in making soft drink cans |
MnO2 used in dry cell | Fe and Mn alloy |
 |
 |
 |
Iron (26Fe )
What are the uses of IORN ?
1-manufacturing of reinforced Concrete.
2-Electricity pylons
3-Knives industry
.4-Pipes guns and cannons industry.
5-Surgical tools

6-As a catalyst in the ammonia industry (Haber-Bosch method).
7-Catalyst for Converting water gas-to-liquid fuel (Fisher -Troposch method). Water gas: is a mixture of hydrogen and carbon monoxide
8- in making magnets
Cobalt 27Co
1) It looks like iron GR? because both of them can be magnetized and used in making of magnets and 2) Cobalt is used in making dry batteries industry in modern cars.
| cobalt used in making magnet | cobalt use in dry batteries |
|
|
|
3)It has twelve isotopes; most important one is cobalt 60 GR? – Because it produces gamma rays that has high ability for penetrating power
3) What are the Uses of Cobalt isotope 60 ?
1-Food preservation processes. 2- Detection of the quality of the industrial products 3- In medicine in diagnosis and treatment tumors 4-To detect cracks and welding connections
Nickel 28Ni
What are the uses of Nickel?
1-Making nickel – cadmium battery that can be recharged. 2-Making Nickel steel alloys which are hard and resist rust and the effect of acids. 3- Making nickel-chrome alloys which are used in heating coils and electrical furnaces ,GR? – Because they resist corrosion at high temperature 4- It is used in painting metal, GR?.
-To protect it from oxidation and rust and give them a beautiful appearance.
5- divided nickel as catalyst is used in the hydrogenation process of oils.
| heating coil | electric furnace | Ni Cd battery |
|
|
|
 |
Copper 29Cu
It is the first discovered metal Uses:
1-It is used in making the alloy of copper with tin (Bronze).
2- Alloy of copper with Zinc (Brass alloy ) used in plating of Iron door handles 2-Making electrical cables, GR?
-Because it is good conductor of electricity. 3-It is used in alloy coins industry

The most important copper compounds 1-Copper II sulphate (CuSO4) :
used as an insecticide and fungicide in the water purification
2- Fehling solution It is used in the detection of glucose where the color turns from blue to orange.

Zinc 30Zn
What are the uses of zinc ?
It is used in galvanizing other metals GR?
-To protect them from rusting what are The most important compounds of Zinc ?
1-Zinc oxide ZnO It is used in paints, rubber and cosmetics industry
2-Zinc sulphide ZnS : used in making a- illuminated paints b- X-ray screens.










