Atom components
Understanding Atom Components: Quiz and Explanations
Introduction: Explore the fundamental components of atoms through an engaging quiz accompanied by detailed explanations. Test your knowledge on atom structure, isotopes, energy transformations, and more, while gaining insights into the intricate world of atomic physics.
Atom components
Matter is composed of atoms. These atoms show the physical and chemical properties of the matter.
Discovery of electrons
By the end of the nineteenth century:
-Scientists considered that electrons are from the main components of the atom
– Electrons are particles with a very small mass
– Electrons have a negative charge and rotate around the nucleus.
– The atom is electrically neutral.
-Because the atom has protons particles carrying positive charges equal to the negative charges of the electrons.
Atomic Models of Rutherford’s (1911) and Bohr’s(1913)

According to the experiment of Rutherford’s and Bohr’s theories ,the atomic structure became as shown in the following:
Rutherford atomic Model
- -In the center of the atom There is a nucleus:
- Tiny and positively charged.
- Relatively heavy in which the mass of the atom is concentrated.
- -Negative electrons rotate around the nucleus at a relatively far distance.
- -Most of the atomic volume is space.
- – The volume of the nucleus is very small relative to the atom’s volume.
- The nucleus diameter=10-6 nm,while the atom’s diameter=1×10-10 nm
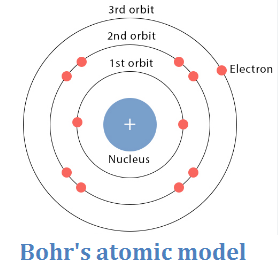
Bohr’s atomic model
- The negatively charged electrons rotate around the nucleus in certain fixed orbits called energy levels.
- Each energy level is occupied by a certain number of electrons that can’t be increased
Discovery of proton (1919)
Rutherford proved that the nucleus of atom contains positively charged particles called protons.
Discovery of neutron (1932)
Nevil Sidgwick discovered that the nucleus contains neutral particles that are called neutrons.
The mass of the neutron is nearly similar to the mass of a proton.
Notes
- The atom’s mass is concentrated in the nucleus, because the mass of electrons is too small (negligible) compared to the mass of protons and neutrons
- The mass of proton=1800 times of the electron mass.
- The atom is electrically neutral, because the number of positive charged protons in the nucleus equals to the number of negative charged electrons rotating around the nucleus.
Explanation and description of the nucleus of the atom of ant element:
Mass number:
- It is Number of protons + Number of neutrons inside the nucleus
- Symbol of Mass number: A
Atomic number:
-It is Number of protons=Number of electrons (in the neutral atom)
Symbol of atomic number: Z
Neutrons number: N
It is Mass number-Number of protons =
-Any element can be represented as follows:

Nucleons are the protons and the neutrons inside the nucleus.
Examples
Write the nucleus symbol of aluminum atom, if its nucleus contains 13 protons and 14 neutrons.
Answer:
Atomic number:13
Mass number :27
Isotopes
Isotopes are atoms of the same element that have the same atomic number (Z), but differ in mass number (A) due to the difference in the number of neutrons inside their nuclei.
The isotopes have the same chemical properties , because they have similar number of electrons and the same electron configuration around the nucleus.
Most elements in the periodic table have more than one isotope.
Examples
Carbon has 3 isotopes
12 13 14
C C C
6 6 6
Isotopes of the same element have the same atomic number
Hydrogen isotopes:

Hydrogen is the simplest element in nature , it has three isotopes, shown in the following table
isotopes of hydrogen
Protium :1H
Atomic number =1.
Mass number =1.
number of Neutrons=1-1= zero
Deutrium:2H
Atomic number =1.
Mass number =2.
number of Neutrons=2-1= 1.
Tritium: 3H
Atomic number =1.
Mass number =3.
number of Neutrons=3-1= 2.
It is clear from the previous that:
The atomic number equals the mass number in protium nucleus, because it doesn’t contain neutrons.
Number of neutrons equals:
– The number of protons in deuterium nucleus.
– Double the number of protons in tritium nucleus.
Examples
Oxygen isotopes:
Oxygen element has three isotopes,shown in the following table:
|
Isotope |
16O | 17O | 18O |
|
No.of protons |
8 |
8 |
8 |
|
No.of nucleons |
16 |
17 |
18 |
|
No.of neutrons |
Note:
It is impossible for the isotope H to exist naturally as the electrical repulsion forces between the protons will not be offset by attraction forces between protons and neutrons as the nucleus does not contain neutrons
Atomic Mass unit amu (u):
- Masses of atomic isotopes can’t be measured in kg, because their masses are too small.
- So, they are measured in atomic mass unit amu (u),
- Where Atomic masses of elements can be identified by knowing the relative atomic masses of their isotopes and the ratio of the presence of each of them.
Examples:
Calculate the atomic mass of copper, if it is found in nature in the form of two isotope 63Cu (69.09 %) and 65Cu (30.91%) [ 63Cu =62.9298 ,65Cu=64.9278 amu)]
Solution:
The contribution of copper-63 in the atomic mass
=62.9298 x 69.09/100
=43.4782 u
The contribution of copper-65 in the atomic mass
=64.9278 x 30.91/100 = 20.069 u
The percentage of copper
Cu=43.4782 + 20.069 =63.55 u
The atomic mass of copper =63.55 u.
2) A sample of lithium contains two isotopes, the first is lithium-6 and its atomic mass is 6.01572 u and the second is lithium-7 and its atomic mass is 7.016 u
Calculate the atomic mass of lithium element Li, if the percentage of lithium-6 found in the sample is 7.42%
Solution:
The percentage of lithium-7 in the sample:
Li%= 100-7.42=92.58%
The contribution of lithium-6 in the atomic mass:
=6.01572 x 7.42/100=0.4464u
The contribution of lithium-7 in the atomic mass:
=7.016 x 92.58/100 = 6.4954u
The atomic mass of lithium element:
=0.4464 + 6.4954=6.9418u
Relation between Mass and energy
Einstein s Equation
Equation illustrates the relation between the transformation (conversion) of a mass of a substance
Calculation of the produced energy from the transformation of a mass of a substance (estimated in kilograms kg) to energy (estimated in Joules J).
Einstein s Equation
E = m * C2
E = energy(J)
m= Transformed mass (Kg)
C2 = Square of Light speed = (3 x 108 m/s)2
Calculation of the produced energy from the transformed mass and energy (estimated in atomic mass unit u) to energy (estimated in million electron volt unit MeV)
By using the following equation:
E = m x 931
E = energy (MeV)
m =mass (u)
931= constant value
Note:
1 eV= 1.6 x 10-19 J
1 MeV=1 x 106 eV
1 MeV= 1.6 x 10-13 J
Examples:
Calculate the amount of energy produced from the transformation of 5g of a substance, estimated in:
(1)Joule (J).
(2) Million electron volt(MeV).
Solution
To covert gram to Kg divide by 1000
m=5/1000 = 0.005
E(J) = m c2
E(J) = 0.004 x (3×108)2
=4.5 x 1014
To convert g to u divide by 1.66 x10-24
m=5/(1.66 x 10-24 )
m=3.012 x1024
(2)E(MeV)=m x 931
E(MeV) =3.012 x1024 x 931
=2.8 x 1027 MeV
-To confirm the results divide the value of energy by unit ( on
E= 4.5 x 1014 / 1.6 x 10-13
E= 2.8 x 1027 MeV
②Calculate the amount of energy (in Joule) produced from the transformation of 25% of 1.4 g of a radioactive substance to energy.
Solution
m= 1.4 x 25/100=0.35
E=m.c2
=0.35/1000 x(3×108)2
=3.15 x 1013 J
③Calculate the mass (in kg) required to produce an amount of energy equals 190 MeV
Solution
m(u) =E/931
=190/931=0.2u
To convert from u to Kg multiply by 1.66 x 10-27
m(Kg)= 0.2 x 1.66 x 10-27
=3.32 x 10-24 Kg
Another solution
E(J) =190 x 1.6×10-13
m(kg) = E/C2
=3.04 x10-11 / (3×108)2
=3.3×10-28 Kg
Quiz: Understanding Atom Components
- What did scientists discover about electrons by the end of the nineteenth century?
- Scientists considered electrons as one of the main components of the atom.
- Electrons have a very small mass.
- Electrons have a negative charge and rotate around the nucleus.
- The atom is electrically neutral due to the balance between positively charged protons and negatively charged electrons.
Explanation: Scientists by the end of the nineteenth century discovered that electrons are negatively charged particles with a very small mass orbiting around the nucleus. This led to the understanding that atoms are electrically neutral due to the balance between the positive charge of protons in the nucleus and the negative charge of electrons orbiting around it.
- Describe Rutherford’s atomic model.
- The nucleus of the atom is tiny and positively charged, containing most of the atom’s mass.
- Negatively charged electrons orbit around the nucleus at a relatively far distance.
- Most of the atomic volume is empty space.
- The nucleus diameter is much smaller than the atom’s diameter.
Explanation: Rutherford’s atomic model proposed that atoms have a nucleus at the center, which is positively charged and contains most of the atom’s mass. Electrons orbit around the nucleus in relatively far orbits, with most of the atomic volume being empty space.
- Explain Bohr’s atomic model.
- Electrons rotate around the nucleus in certain fixed orbits called energy levels.
- Each energy level can only be occupied by a specific number of electrons.
- This model provided a more structured understanding of electron arrangement compared to Rutherford’s model.
Explanation: Bohr’s atomic model introduced the concept of electrons orbiting around the nucleus in specific energy levels, unlike Rutherford’s model where electrons moved in random orbits. This model helped explain atomic spectra and electron arrangement in atoms.
- What was discovered in 1919 regarding the components of the atom?
- Rutherford proved the existence of positively charged particles called protons in the nucleus.
Explanation: Rutherford’s experiment in 1919 provided evidence for the existence of positively charged particles within the nucleus of an atom, which were named protons.
- Who discovered the neutron and when?
- Neutrons were discovered by James Chadwick in 1932.
Explanation: Neutrons were discovered by James Chadwick in 1932 through his experiments with beryllium and alpha particles.
- Define Mass Number (A) and Atomic Number (Z).
- Mass Number (A): It is the sum of the number of protons and neutrons inside the nucleus.
- Atomic Number (Z): It represents the number of protons (or electrons in a neutral atom).
Explanation: The mass number (A) indicates the total number of nucleons (protons and neutrons) in an atom’s nucleus, while the atomic number (Z) represents the number of protons (and electrons in a neutral atom), defining the element.
- Calculate the nucleus symbol of an aluminum atom with 13 protons and 14 neutrons.
- Atomic number (Z) = 13
- Mass number (A) = 13 (protons) + 14 (neutrons) = 27
- Therefore, the nucleus symbol is ^{27}_{13}Al.
Explanation: The nucleus symbol is written in the form A/Z Element, where A represents the mass number and Z represents the atomic number. For aluminum, with 13 protons and 14 neutrons, the symbol is ^{27}_{13}Al.
- Explain what isotopes are and provide an example.
- Isotopes are atoms of the same element with the same atomic number (Z) but different mass numbers (A) due to varying numbers of neutrons.
- An example is carbon, which has isotopes carbon-12, carbon-13, and carbon-14.
Explanation: Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons. Carbon, for instance, has isotopes carbon-12, carbon-13, and carbon-14, which all have six protons but differing numbers of neutrons.
- Calculate the atomic mass of copper given two isotopes: 63Cu (69.09%) and 65Cu (30.91%).
- Atomic mass = (Mass of 63Cu * % abundance of 63Cu) + (Mass of 65Cu * % abundance of 65Cu)
- Atomic mass = (62.9298 * 69.09/100) + (64.9278 * 30.91/100) = 63.55 u
Explanation: The atomic mass of copper is calculated by summing the contributions of each isotope’s mass weighted by its relative abundance.
- Using Einstein’s equation, calculate the energy produced from the transformation of 5g of a substance to energy.
- E=mc2
- m=0.005 kg m=0.005kg (since 1 g=0.001 kg1g=0.001kg)
- c=3×108 m/sc=3×108m/s
- E=0.005×(3×108)2=4.5×1014 JE=0.005×(3×108)2=4.5×1014J
Explanation: Einstein’s equation describes the conversion of mass to energy, where m is the mass, c is the speed of light, and E is the energy produced.
Multiple Choice Quiz: Understanding Atom Components
- What did scientists discover about electrons by the end of the nineteenth century?
a) Electrons have a positive charge
b) Electrons have a very large mass
c) Electrons have a negative charge and rotate around the nucleus
d) Electrons are stationary inside the nucleus
Explanation: c) By the end of the nineteenth century, scientists discovered that electrons have a negative charge and they orbit around the nucleus of an atom.
- Which of the following statements best describes Rutherford’s atomic model?
a) Electrons are evenly distributed throughout the atom
b) The nucleus is negatively charged and contains most of the atom’s mass
c) Electrons orbit the nucleus in specific energy levels
d) The nucleus is positively charged and contains most of the atom’s mass
Explanation: d) Rutherford’s atomic model proposed that the atom has a small, positively charged nucleus at its center, containing most of the atom’s mass.
- Who discovered the neutron?
a) Ernest Rutherford
b) James Chadwick
c) Niels Bohr
d) J.J. Thomson
Explanation: b) James Chadwick discovered the neutron in 1932 through his experiments with beryllium and alpha particles.
- What is the mass number of an atom?
a) Number of protons
b) Number of neutrons
c) Sum of protons and neutrons
d) Number of electrons
Explanation: c) The mass number of an atom is the sum of the number of protons and neutrons in its nucleus.
- Isotopes are atoms of the same element that have:
a) Different atomic numbers
b) Different numbers of protons
c) Different mass numbers
d) Different electron configurations
Explanation: c) Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons, leading to different mass numbers.
- Which equation describes the relationship between mass and energy?
a) E=mc
b) E=mc2
c) E=m2c3
dE=mc3
Explanation: b) Einstein’s equation E=mc2 describes the relationship between mass and energy, where E is energy, m is mass, and c is the speed of light.
- Calculate the atomic mass of copper if it is found in nature as 69.09% 63Cu (62.9298 amu) and 30.91% 65Cu (64.9278 amu).
a) 63.55 amu
b) 65.37 amu
c) 61.84 amu
d) 67.22 amu
Explanation: a) The atomic mass is calculated by multiplying the mass of each isotope by its relative abundance and summing the results.
- What is the relationship between the atomic number and the number of protons?
a) They are equal
b) The atomic number is greater than the number of protons
c) The atomic number is less than the number of protons
d) They are unrelated
Explanation: a) The atomic number of an element is equal to the number of protons in the nucleus of its atom.
- Which of the following best describes Bohr’s atomic model?
a) Electrons move randomly around the nucleus
b) Electrons orbit the nucleus in specific energy levels
c) The nucleus is evenly distributed throughout the atom
d) The nucleus contains both positive and negative charges
Explanation: b) Bohr’s atomic model proposed that electrons orbit the nucleus in specific energy levels, unlike Rutherford’s model where electrons moved randomly.
10-What is the charge of a neutron?
a) Positive
b) Negative
c) Neutral
d) Variable
Explanation: c) Neutrons have no net electric charge, making them neutral particles.
These multiple-choice questions cover various aspects of atom components, including electrons, protons, neutrons, isotopes, atomic models, and their properties.
11-Which of the following statements regarding electrons is correct?
a) Electrons are located within the nucleus of an atom.
b) Electrons have a positive charge.
c) Electrons orbit around the nucleus of an atom.
d) Electrons have a mass equal to that of protons.
Explanation: c) Electrons are negatively charged particles that orbit around the nucleus of an atom in specific energy levels or shells.
12-What did Rutherford’s experiment with alpha particles demonstrate?
a) The nucleus is positively charged.
b) Electrons are located in fixed orbits.
c) Atoms are mostly empty space.
d) Protons are located outside the nucleus.
Explanation: c) Rutherford’s experiment showed that atoms are mostly empty space with a dense, positively charged nucleus at the center.
13-Which subatomic particle has a mass approximately equal to that of a proton?
a) Neutron
b) Electron
c) Positron
d) Meson
Explanation: a) Neutrons have a mass approximately equal to that of protons. Both particles have a mass of about 1 atomic mass unit (amu).
14-What is the total number of protons and neutrons in an atom with a mass number of 23 and an atomic number of 11?
a) 34
b) 12
c) 11
d) 23
Explanation: a) The mass number represents the total number of protons and neutrons in an atom. So, for an atom with a mass number of 23, it has 11 protons (given by the atomic number) and 23 – 11 = 12 neutrons.
15-Which of the following isotopes of hydrogen has two neutrons?
a) Protium
b) Deuterium
c) Tritium
d) Helium
Explanation: b) Deuterium is the isotope of hydrogen with one proton and one neutron. Tritium has two neutrons, and protium has no neutrons.
16-What unit is used to measure atomic masses?
a) Gram (g)
b) Kilogram (kg)
c) Atomic mass unit (amu)
d) Meter (m)
Explanation: c) Atomic masses are typically measured in atomic mass units (amu), which is a unit of mass used to express the mass of atoms or molecules.
