The graduation of the representative elements properties :Atomic Radius,The effective nuclear charge
The graduation of the representative elements properties
Explore the concepts of atomic and ionic radii, effective nuclear charge, and size variations of ions in chemistry. Learn how these factors impact the properties of elements and compounds
The chemical properties and some of physical properties of the elements depend on their electronic configuration and specially on the valence electrons (the electrons of the outermost level).
The atomic radius
- The bond length in the covalent compounds differs than in the ionic compounds
- The atomic radius is a measure of the size of an atom.
- Atomic radius is influenced by factors such as the number of energy levels (shells), the effective nuclear charge, and electron-electron repulsion.

Ionic Radius:
- In ionic compounds, atoms can gain or lose electrons to become ions.
- The size of an ion, called the ionic radius, can be different from the atomic radius.
- Cations (positively charged ions) are smaller than their parent atoms because they lose electrons.
- while anions (negatively charged ions) are larger because they gain

How to calculate atomic radius
Atomic radius is not directly measured by the distance between the nucleus and the farthest electron GR?
-Because it is impossible to determine the precise location of an electron around the nucleus (depending on the wave mechanics theory)

There are a few common methods and principles used to estimate atomic radii:
Covalent Radii:
Atomic radii can be estimated based on the distance between the nuclei of two covalently bonded atoms in a molecule.
Atomic radius can not be defined or can not be physical measured why?
Atomic radius cannot be precisely defined or physically measured
because electrons in atoms do not have well-defined distinct boundaries like solid objects. Instead, electrons are described by probability distributions in the form of electron clouds or electron density functions.
How to calculate the atomic radius by covalent bond length?
It can be estimated from its covalent bond length in a molecule.
The covalent bond length is the distance between the center of the two nuclei of two bonded atoms.
By knowing Covalent bond length(2r) we can calculate :
1)Atomic radius
Calculate the Atomic Radius:
The formula is:
Covalent bond length=sum of the two atomic radii of the molecule of the two atom
The atomic radius (r)=bond length in diatomic molecule(2r)/2

Example:
The experimental internuclear distance in Cl2 molecule is 1.98 Å. The covalent radius of chlorine is calculated as below.

Here’s a table that includes the bond length and covalent atomic radius for molecules H2, F2, Cl2, Br2, and I2. The bond lengths and covalent radii are approximate values and may vary slightly depending on the specific data source and method used.
Molecule Bond Length (Å) Covalent Atomic Radius (Å)
H2 0.60 0.30 (for hydrogen)
F2 1.28 0.64 (for fluorine)
Cl2 1.99 0.99 (for chlorine)
Br2 2.28 1.14 (for bromine)
I2 2.67 1.33 (for iodine)
Example:
The bond length in chlorine molecule Cl-Cl is 1.98 A
The bond length between carbon and chlorine atoms C-Cl in carbon tetrachloride CCl4 is 1.76 A Calculate the atomic radius of carbon
Solution:
The atomic radius of chlorine = bond length in chlorine molecule Cl2/2
The atomic radius of chlorine=1.98/2=0.99 A
The atomic radius of carbon =the (C-Cl)bond length – The atomic radius of chlorine
The atomic radius of carbon =1.76 – 0.99 = 0.77 A
Example:
The bond length in hydrogen molecule (H-H)=0.6 A
The bond length of nitrogen N2=1.4 A
The bond length of nitric oxide molecule NO=1.36 A
Calculate :
- The bond length in oxygen molecule O2
- The bond length (O-H) in water molecule H2O
Solution:
(1)The atomic radius of nitrogen= bond length of N2 molecule/2
The atomic radius of nitrogen=1.4/2=0.7 A
The atomic radius of Oxygen = the (N-O) bond length – the atomic radius of nitrogen
The atomic radius of Oxygen=1.36 – 0.7= 0.66 A
The bond length in oxygen molecule = 2 x atomic radius of oxygen
The bond length in oxygen molecule=2 x 0.66=1.32A
(2) The atomic radius of hydrogen = bond length of hydrogen H2 molecule/2
The atomic radius of hydrogen=0.6/2=0.3A
The bond length (O – H) =the atomic radius of oxygen + the atomic radius of hydrogen
The bond length (O – H)=0.66 +0.3=0.96 A
How to estimate ionic bond length by using ionic radius
2) by Ionic bond length
- The sum of the ionic radii of the cation and anion
- The distance between the centers of the nuclei of two bonded ions.
- The ionic radius depends on the number of electron gained or lost to form ions

Calculate the Estimated Bond Length:
Add the ionic radii of the cation and anion to estimate the bond length.
Estimated Bond Length = Ionic Radius (cation) + Ionic Radius (anion)
Example
Identify ionic bond length in NaCl
For the sake of this example, let’s assume:
Ionic Radius (Na⁺) = 0.95 Å (angstroms)
Ionic Radius (Cl⁻) = 1.81 Å
Calculate the Estimated Bond Length:
Estimated Bond Length = Ionic Radius (Na⁺) + Ionic Radius (Cl⁻)
Estimated Bond Length = 0.95 Å + 1.81 Å
Estimated Bond Length of NaCl = 2.76 Å
Example:
Ionic Radius of Lithium Ion (Li⁺): Approximately 0.76 angstroms (Å)
Ionic Radius of Sodium Ion (Na⁺): Approximately 0.95 angstroms (Å)
Calculate the ionic bond length in lithium chloride formula unit:
In sodium chloride (NaCl), The sodium ion (Na⁺) has an ionic radius of 0.95 angstroms (Å), and the chloride ion (Cl⁻) has an ionic radius of 1.81 angstroms (Å).
Calculate the ionic bond length in LiCl formula unit
solution
Ionic Radius of Lithium Ion (Li⁺): Approximately 0.76 angstroms (Å)
Ionic Radius of Chloride Ion (Cl⁻): Approximately 1.81 angstroms (Å)
The ionic bond length in LiCl is determined by the distance between the centers of the Li⁺ and Cl⁻ ions. So, you can calculate it as follows:
Estimated Bond Length (LiCl) = Ionic Radius (Li⁺) + Ionic Radius (Cl⁻)
Estimated Bond Length (LiCl) = 0.76 Å + 1.81 Å
Estimated Bond Length (LiCl) = 2.57 Å
The effective nuclear charge (Zeff) concept
- It refers to the net positive charge experienced by an electron in an atom, taking into account the actual nuclear charge ,the shielding effect of inner electrons.
- In other words, Zeff is the “apparent” or “effective” charge that an electron in the outermost energy level (valence shell) of an atom experiences.
- A greater effective nuclear charge leads to a smaller atomic radius, while a smaller effective nuclear charge results in a larger atomic
- Zeff is usually less than the actual nuclear charge (Z) because inner electrons partially shield the outer electrons from the full force of the nucleus.

- Mathematically, it can be expressed as: Zeff = Z – Shielding or Screening Effect
- The greater the shielding effect, the lower the Zeff an outer electron will experience, and vice versa.
- As you move across a period from left to right, Zeff increases, leading to a higher ionization energy and a smaller atomic size.
- As you move down a group (a column) in the periodic table, Zeff generally remains relatively constant, as the number of inner electron shells increases, providing greater shielding for the outer electrons.

Actual Nuclear Charge (Z)
- Every atom has a positively charged nucleus consisting of protons (positively charged) and neutrons (uncharged).
- The actual nuclear charge (Z) is the number of protons in the nucleus.
- It represents the positive charge that attracts electrons.
- Nuclear charge, denoted as “Z,” represents the total positive charge in the nucleus of an atom.
- Z does not consider the influence of electron-electron repulsions on the outermost electrons.
Shielding or Screening Effect:
- Within an atom, there are inner electrons in lower energy levels (closer to the nucleus).
- These inner electrons repel the outer electrons due to their negative charge.
- This electron-electron repulsion partially counteracts the attraction between the outer electrons and the nucleus.

The graduation of atomic radius in the periodic table
Across a period (from left to right):
- The atomic radius generally decreases as you move from left to right across a period.
- This is because each element in the same period has the same number of energy levels (shells), but the number of protons in the nucleus increases, creating a stronger positive charge that pulls the electrons closer to the nucleus.
- Electrons are added to the same energy level (shell) as you move across the period, so the increase in the effective nuclear charge outweighs the increase in electron shielding, leading to a smaller atomic radius.

Down a group (from top to bottom):
- The atomic radius generally increases as you move down a group.
- This is due to the addition of more energy levels (shells) with each successive element in the group.
- Additionally, the number of electron shells increases, resulting in increased electron shielding, which reduces the effective nuclear charge experienced by the outermost electrons.
Across a Period (Left to Right):
- As you move from left to right across a period, the atomic radius generally decreases.
- This is because the effective nuclear charge (Zeff) increases, meaning there are more protons in the nucleus, which exerts a stronger attraction on the electrons. As a result, the electrons are pulled closer to the nucleus, making the atomic radius smaller.
The graduation of atomic radius in the periodic table
The graduation of atomic radius in the periodic table reveals several key conclusions and trends:
Atomic Radius Decreases Across a Period: As you move from left to right across a period (horizontal row), the atomic radius generally decreases. GR?
– Because of the increasing effective nuclear charge (Zeff) as you add more protons to the nucleus.
-The greater attraction between the positively charged nucleus and the negatively charged electrons causes the electrons to be pulled closer to the nucleus, resulting in a smaller atomic radius.
Atomic Radius Increases Down a Group GR?
As you move down a group (vertical column) in the periodic table, the atomic radius generally increases.
This is due to the addition of energy levels (electron shells) as you move down the group. Electrons in higher energy levels are, on average, farther from the nucleus, resulting in a larger atomic radius.
Mention which atom is larger in siz in the following pairs of atoms with an explanation and F , Ba and Be
N (Nitrogen) and F (Fluorine):
Nitrogen (N) is larger than Fluorine (F).
Explanation:
- Both nitrogen and fluorine are in the same period (period 2) of the periodic table.
- As you move from left to right across a period, the atomic radius generally decreases.
- Nitrogen has one more electron shell (energy level) than fluorine, which means that the outermost electrons in nitrogen are farther from the nucleus.
- This results in a larger atomic radius for nitrogen compared to fluorine.
Ba (Barium) and Be (Beryllium): Barium (Ba) is larger than Beryllium (Be).
Explanation:
- Barium and beryllium are in the same group (Group 2, also known as the alkaline earth metals) of the periodic table.
- As you move down a group, the atomic radius generally increases. Barium is located below beryllium in the periodic table, so it has more energy levels (electron shells).
- This leads to a larger atomic radius for barium compared to beryllium.
- In summary, nitrogen is larger than fluorine because nitrogen is in a higher period, and barium is larger than beryllium because barium is lower down in the same group of the periodic table. The periodic trends for atomic radius explain these differences in size.
The relation between the radii of atoms and their ions: in metals
In metals, the relationship between the radii of atoms and their ions plays a crucial role in understanding the behavior of metals in chemical reactions and the formation of ionic compounds. Here are the key points regarding this relationship:
Cations (Positive Ions):
- When a metal atom loses one or more electrons to become a cation (positively charged ion), it becomes smaller in size compared to the neutral atom from which it originated.

GR:The size of the metal cation in the ionic compound is smaller than the atomic radius of the neutral metal atom due to electron loss.GR?
– Because, with the loss of electrons, there are fewer negatively charged electrons surrounding the nucleus. As a result, the remaining electrons are more strongly attracted to the positively charged nucleus, pulling them closer to the center of the atom.
The more electrons a metal atom loses, the smaller the resulting cation. Cations are smaller than their parent metal atoms.
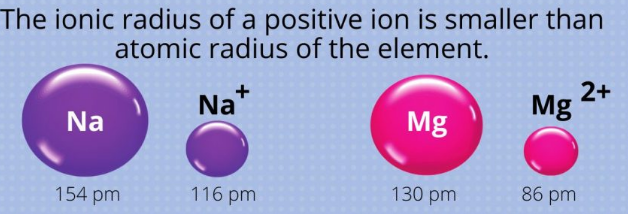
Anions (Negative Ions):
- Nonmetals tend to gain electrons during chemical reaction to form negative ions GR?
- As the number of negative electrons in the anion is larger than the number of positive protons inside the nucleus so the repulsion force between electrons increases due to increasing the number of electrons without increasing the number of protons(nuclear charge) leading to increase the size
- The negative ion radius is larger than its atomic radius
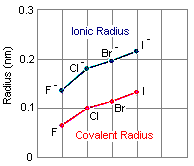
Example
Let’s consider a few examples to illustrate the relationship between the radii of metal atoms and their ions:
Sodium (Na):
- The neutral sodium atom has an atomic radius of approximately 86 A.
- When sodium loses one electron to form a sodium cation (Na⁺), it becomes significantly smaller.
- The ionic radius of the sodium cation decreases to 98 A due to the effective nuclear (positive) charge of the ion increases.
- This reduction in size is due to the removal of an electron and the increased attraction between the remaining electrons and the nucleus.
Iron (Fe):
- Iron can form cations with different charges, such as Fe²⁺ and Fe³⁺. When iron loses electrons to form these cations, the resulting ions are smaller than the neutral iron atom.
- The ionic radius of neutral Fe atom:1.17A
- The ionic radius of Fe²⁺ is approximately 0.75A.
- The ionic radius of Fe3+ (ferric ion)0.6A.
- When iron loses electrons to form a positive ion (cation), the electron cloud becomes smaller, leading to a decrease in the ionic radius compared to the neutral atom.
- This is because the loss of electrons reduces the electron-electron repulsion, allowing the remaining electrons to be pulled closer to the nucleus.
- Atomic radius of neutral iron > Ionic radius of Fe2+ > Ionic radius of Fe3+
Anions (Negative Ions):
- Nonmetals typically form anions (negatively charged ions) by gaining electrons.
- When a nonmetal atom gains one or more electrons to become an anion, it becomes larger in size compared to the neutral atom from which it originated.
- The addition of electrons increases the electron-electron repulsion, causing the electrons to spread out, and this results in a larger atomic radius for the anion compared to the neutral atom.
- Anions formed from nonmetals are larger than their parent nonmetal atoms.
The relation of radius of nonmetal atom and its ion
Anions (Negative Ions) – Increase in Size:
- Nonmetal atoms can gain one or more electrons to become negatively charged anions.
- When a nonmetal atom gains electrons to form an anion, it becomes larger in size compared to the neutral atom from which it originated.
- The addition of electrons leads to increased electron-electron repulsion, causing the electrons to spread out and occupy larger electron clouds.
- Anions formed from nonmetals are generally larger than their parent nonmetal atoms.

Chlorine (Cl) – Anion Formation:
- Chlorine is a nonmetal element with atomic number 17.
- The neutral chlorine atom has an atomic radius of approximately 0.099 nanometers (nm).
- When chlorine gains one electron to form the chloride ion (Cl⁻), the resulting ion is larger than the neutral chlorine atom.
- The ionic radius of Cl⁻ is approximately 0.181 nm.
- This increase in size is due to the addition of an electron, which increases electron-electron repulsion, causing the electron cloud to expand.
Quiz 1 Ionic Radii and Effective Nuclear Charge
1-True or False: Anions formed from nonmetal atoms are generally smaller in size compared to their neutral parent atoms.
2-Which of the following ions has the largest ionic radius?
- a) Sodium ion (Na⁺) b) Magnesium ion (Mg²⁺) c) Potassium ion (K⁺) d) Aluminum ion (Al³⁺)
3-True or False: As you move down a group (column) in the periodic table, metal atoms tend to form smaller cations.
4-The effective nuclear charge experienced by an ion is influenced by:
- a) The nuclear charge and the number of protons in the nucleus.
- b) The number of electrons and the number of neutrons.
- c) The number of energy levels (shells) in the atom. d) The number of valence electrons.
5-Which of the following ions has the smallest ionic radius?
- a) Chloride ion (Cl⁻) b) Sulfate ion (SO₄²⁻) c) Nitride ion (N³⁻) d) Oxide ion (O²⁻)
6-True or False: The ionic radius of an anion is generally smaller than the atomic radius of its parent neutral atom.
7-Which of the following ions has the highest effective nuclear charge?
- a) Magnesium ion (Mg²⁺) b) Sodium ion (Na⁺) c) Potassium ion (K⁺) d) Lithium ion (Li⁺)
8-In a chemical reaction, a neutral iron atom (Fe) loses three electrons to form the iron(III) ion (Fe³⁺). How does the ionic radius of the iron(III) ion compare to the atomic radius of the neutral iron atom?
9-True or False: The effective nuclear charge of an ion is solely determined by the number of electrons in the ion.
10-When a nonmetal atom gains electrons to form anions, what effect does this have on the ionic radius?
1-Answer: False 2-Answer: c) Potassium ion (K⁺) 3-Answer: False
4-Answer: a) The nuclear charge and the number of protons in the nucleus.
5- Answer: c) Nitride ion (N³⁻) 6-Answer: False 7-Answer: a) Magnesium ion (Mg²⁺)
8-Answer: The ionic radius of the iron(III) ion (Fe³⁺) is smaller than the atomic radius of the neutral iron atom (Fe).
9-Answer: False
10-Answer: The ionic radius increases because the added electrons lead to increased electron-electron repulsion and greater shielding, causing the electron cloud to expand.
Quiz 2 : Ionic Radii and Effective Nuclear Charge (Advanced)
1-True or False: The effective nuclear charge (Zeff) experienced by an ion is always equal to its nuclear charge (atomic number).
2-Which of the following elements, when forming anions, tends to exhibit the largest increase in ionic radius compared to its neutral atom?
- a) Carbon (C) b) Nitrogen (N) c) Oxygen (O) d) Sulfur (S)
3-In a chemical reaction, a neutral nitrogen atom (N) gains three electrons to form the nitride ion (N³⁻). How does the ionic radius of the nitride ion compare to the atomic radius of the neutral nitrogen atom?
4-Which of the following elements is most likely to form the smallest cation when losing electrons? a) Calcium (Ca) b) Potassium (K) c) Magnesium (Mg) d) Sodium (Na)
5-True or False: The effective nuclear charge (Zeff) experienced by an ion is influenced only by the nuclear charge and not by the number of electrons or electron configuration.
6-In a chemical reaction, a neutral oxygen atom (O) gains two electrons to form the oxide ion (O²⁻). How does the ionic radius of the oxide ion compare to the atomic radius of the neutral oxygen atom?
7-Which of the following cations has the highest effective nuclear charge?
- a) Iron(II) ion (Fe²⁺) b) Aluminum ion (Al³⁺) c) Copper(I) ion (Cu⁺) d) Zinc ion (Zn²⁺)
8-True or False: In general, cations formed from metal atoms are larger in size compared to the neutral metal atoms.
9-When a nonmetal atom loses electrons to form cations, what effect does this have on the ionic radius?
10-Compare the ionic radius of the aluminum ion (Al³⁺) to that of the aluminum atom (Al). Which is larger, and why?
1-Answer: False 2-Answer: d) Sulfur (S) 3-Answer: The ionic radius of the nitride ion (N³⁻) is larger than the atomic radius of the neutral nitrogen atom (N). 4-Answer: d) Sodium (Na) 5-Answer: False
6-Answer: The ionic radius of the oxide ion (O²⁻) is larger than the atomic radius of the neutral oxygen atom (O).
7-Answer: b) Aluminum ion (Al³⁺) 8-Answer: False 9-Answer: The ionic radius decreases because the loss of electrons results in a higher effective nuclear charge, causing the remaining electrons to be pulled closer to the nucleus.
10-Answer: The ionic radius of the aluminum ion (Al³⁺) is smaller than the atomic radius of the neutral aluminum atom (Al) because the ion has a higher effective nuclear charge, which attracts the remaining electrons more strongly.
Quiz 3 Ionic Radii and Effective Nuclear Charge (Advanced)
1-True or False: The effective nuclear charge (Zeff) experienced by an ion is the same for all ions of the same element.
2-Which of the following ions has the smallest ionic radius?
- a) Chromium(III) ion (Cr³⁺) b) Nickel(II) ion (Ni²⁺) c) Cobalt(II) ion (Co²⁺) d) Iron(III) ion (Fe³⁺)
3-When a metal atom loses electrons to form a cation, what happens to the effective nuclear charge (Zeff)?
4-True or False: Cations formed from transition metals are generally smaller in size compared to those formed from main-group metals.
5-In a chemical reaction, a neutral sulfur atom (S) gains two electrons to form the sulfide ion (S²⁻). How does the ionic radius of the sulfide ion compare to the atomic radius of the neutral sulfur atom?
6-Which of the following ions has the highest effective nuclear charge?
- a) Copper(II) ion (Cu²⁺) b) Silver(I) ion (Ag⁺) c) Zinc ion (Zn²⁺) d) Lead(II) ion (Pb²⁺)
7-True or False: In general, nonmetal anions are smaller in size than nonmetal cations.
8-When a nonmetal atom gains electrons to form anions, what effect does this have on the ionic radius?
9-Compare the ionic radius of the oxygen ion (O²⁻) to that of the oxygen atom (O). Which is larger, and why?
10-In a chemical reaction, a neutral calcium atom (Ca) loses two electrons to form the calcium ion (Ca²⁺). How does the ionic radius of the calcium ion compare to the atomic radius of the neutral calcium atom?
1-Answer: False
2-Answer: a) Chromium(III) ion (Cr³⁺)
3-Answer: The effective nuclear charge (Zeff) increases.
4-Answer: True
5-Answer: The ionic radius of the sulfide ion (S²⁻) is larger than the atomic radius of the neutral sulfur atom (S).
6-Answer: a) Copper(II) ion (Cu²⁺)
7-Answer: True
8-Answer: The ionic radius increases because the added electrons lead to increased electron-electron repulsion and greater shielding, causing the electron cloud to expand.
9-Answer: The ionic radius of the oxygen ion (O²⁻) is larger than the atomic radius of the neutral oxygen atom (O) because the ion has gained electrons, leading to increased electron-electron repulsion and a larger electron cloud.
10-Answer: The ionic radius of the calcium ion (Ca²⁺) is smaller than the atomic radius of the neutral calcium atom (Ca).
Quiz 4 : Ionic Radii, Effective Nuclear Charge, and Related Concepts (Advanced)
1-True or False: The effective nuclear charge (Zeff) experienced by an ion is solely determined by the number of protons in the nucleus.
2-Which of the following ions has the highest effective nuclear charge?
- a) Aluminum ion (Al³⁺) b) Sodium ion (Na⁺) c) Magnesium ion (Mg²⁺) d) Potassium ion (K⁺)
3-When a nonmetal atom gains electrons to form anions, how does the number of energy levels (shells) in the atom typically change?
4-True or False: The size of ions formed from nonmetals is influenced solely by the number of electrons in the ion.
5-In a chemical reaction, a neutral lithium atom (Li) loses one electron to form the lithium ion (Li⁺). How does the ionic radius of the lithium ion compare to the atomic radius of the neutral lithium atom?
6-Which of the following ions has the smallest ionic radius?
- a) Nitride ion (N³⁻) b) Oxide ion (O²⁻) c) Fluoride ion (F⁻) d) Sulfide ion (S²⁻)
7-True or False: The effective nuclear charge experienced by an ion remains constant across different ionic charge states of the same element.
8-When a metal atom loses electrons to form a cation, what happens to the electron-electron repulsion in the electron cloud?
9-Compare the ionic radius of the iron(II) ion (Fe²⁺) to that of the iron(III) ion (Fe³⁺). Which is larger, and why?
10-True or False: The ionic radius of an anion is always larger than the atomic radius of its parent neutral atom.
1-Answer: False
2-Answer: a) Aluminum ion (Al³⁺)
3-Answer: The number of energy levels increases due to the addition of electrons.
4-Answer: False
5-Answer: The ionic radius of the lithium ion (Li⁺) is smaller than the atomic radius of the neutral lithium atom (Li).
6-Answer: a) Nitride ion (N³⁻)
7-Answer: False
8-Answer: The electron-electron repulsion decreases, leading to a more compact electron cloud. 9-Answer: The ionic radius of the iron(II) ion (Fe²⁺) is larger than that of the iron(III) ion (Fe³⁺) because the former has a lower positive charge and a lower effective nuclear charge. 10-Answer: True
