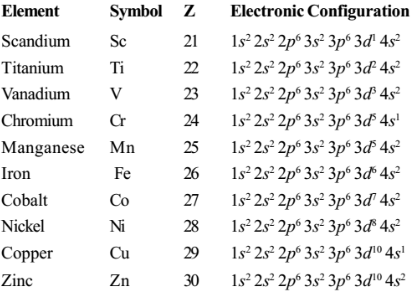
Electronic configuration of First transition series
Electronic configuration of d-block
- General electron configuration of d-block elements is: ns1-2(n-1)d1-10
- The valence configuration for first series transition metals (Groups 3 – 12) is usually 3dn 4s2.

Exceptions
⊗ The first transition series is located in the fourth period after calcium 20Ca [Ar]4s2
⊗The 3d gradually filled by single electron in each orbital till manganese then the pairing of electron occurs in each orbital till zinc 3d10 according Hund’ s rule
⊗The electron configurations for chromium (3d5 4s1) and copper (3d10 4s1). GR?
-This is because 3d and 4s orbitals are very close in energy, and the energy of 3d orbitals drops going across the row.
⊗ For both chromium and copper the configuration having more electrons in in 3d orbitals is of lower energy.
⊗For chromium this is because the difference in 3d and 4s orbital energies is similar to the pairing energy (Electron pairs are of higher energy).
⊗The 3d5 4s1 configuration is of lower energy because this configuration has the maximum number of unpaired electrons for a d-subshell.
⊗For copper (near the end of the transition series) 3d orbital energy has dropped so that 3d orbitals are of lower energy than 4s orbitals
This means the 3d10 4s1 configuration is of lower energy because it has more electrons in 3d orbitals.
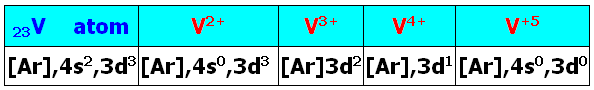
⊗For the transition metal atoms, the total number of valence electrons equals the number of the column (group) in the periodic table (counting from the left).
⊗For transition metal ions having charge ≥ +2, the number of d electrons equals the total number of valence electrons minus the charge on the ion. No. of d electrons=no. of valence electrons –the charge of ion
This is because:
- orbitals in the 3d and 4s subshells are of similar energy.
- In transition metal atoms the 4s subshell is of lower energy than the 3d subshell.
- In transition metal ions of charge ≥ +2, 3d is of lower energy than 4s.
- In transition metal ions of charge ≥ +2, all valence electrons in the d-subshell.
Therefore:
- Ni (Group 10) has 10 valence electrons and Ni2+ is d8
- Fe (Group 8) has 8 valence electrons and Fe3+ is d5
- Ti (Group 4) has 4 valence electrons and Ti3+ is d1
Note: The atom become more stable(less energy)when the d sublevel :
- 1)half filled (d5 )
- 2)completely filled with electron (d10)
- 3-d is empty d0
An element x its electronic configuration is:
[54Xe]6S2,…… 5d5 determine:
a)The transition series order ……
(answer: third transition series )
b)period No.:……
(answer: 6 )
c)column No.:……(answer: 7 ) Rhenium. Atomic number,
75 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d5 6s2.
Question: choose the correct answer :
The outer electron configuration of first transition series is:
. Then value of is: (3,4,5,6) (answer:4
Quiz1 : please click : [HDquiz quiz = “17”]
